Herbicide resistance in downy brome in the dryland wheat production region of northeastern Oregon


Downy brome (Bromus tectorum L.) is a challenging weed to control in dryland wheat production systems in northeastern Oregon. Growers in the region predominantly rely on postemergence acetolactate synthase (ALS) inhibitors (Group 2 herbicides) to control downy brome in wheat. However, the repeated and prolonged use of these herbicides has led to the evolution of resistant downy brome populations. This article presents findings from a survey conducted in 2021 and 2022, which evaluated downy brome management practices and the prevalence of herbicide resistance in this species across wheat fields in the region. Earn 0.5 CEUs in Integrated Pest Management by taking the quiz.
Downy brome has long been a major problem in dryland wheat-based cropping systems in the Pacific Northwest, particularly in northeastern Oregon. Native to the Mediterranean region, this species has become a globally distributed weed, successfully colonizing various non-crop disturbed areas and cultivated fields. Downy brome is a predominantly self-pollinated, winter annual grass that reproduces exclusively by seeds (Hulbert, 1955; Kao et al., 2008). It typically germinates in the fall with occasional flushes in early spring. This fast-growing and highly competitive weed can produce up to 1,350 seeds per plant (San Martín et al., 2021). Seedling emergence is most successful at the soil surface while deeper burial significantly inhibits germination, making no-tillage systems particularly conducive to downy brome infestations. If left uncontrolled, downy brome can reduce wheat yields by as much as 92% (Rydrych & Muzik, 1968).

Managing downy brome in wheat is challenging due to biological similarities, including emergence and maturation timing, that limit herbicide options. The most common postemergence herbicides used for downy brome control in winter wheat are acetolactate synthase (ALS) inhibitors, also known as Group 2 herbicides. Currently, growers have five ALS herbicide options: Osprey (mesosulfuron), Outrider (sulfosulfuron), Olympus (propoxycarbazone), PowerFlex HL (pyroxsulam), and Beyond (imazamox). Among these, Beyond is specifically used with Clearfield wheat and will kill non-Clearfield and CoAXium wheat varieties. Extensive reliance on these herbicides over the past two decades has led to the selection of ALS-resistant populations in wheat fields. Additionally, the photosystem II (PSII) inhibitor metribuzin (Group 5) is labeled for postemergence use in wheat. To provide growers with another option for winter annual grass control, the CoAXium Wheat Production System was introduced in 2018 in the U.S. Pacific Northwest. This system allows postemergence applications of quizalofop, an acetyl-CoA carboxylase (ACCase) inhibitor (Group 1), marketed as Aggressor AX, for selective control of grasses, including downy brome. Nonetheless, ALS inhibitors continue to be important tools for downy brome management, and understanding the distribution and patterns of resistance is critical.
To better understand the extent of resistance in downy brome, we conducted surveys in 2021 and 2022 to assess downy brome management practices among wheat growers in northeastern Oregon. Additionally, we collected seeds from 49 downy brome populations in wheat fields across the region and screened them for resistance to commonly used herbicide modes of action in dryland wheat production systems.
Downy brome management survey
Surveys were conducted in 2021 and 2022 to assess management practices for downy brome in wheat fields across northeastern Oregon. With the help of three county extension agents, 28 growers were identified with fields infested with downy brome in Gilliam, Morrow, Sherman, Umatilla, and Wasco counties. The survey included four key questions: (1) crop rotation, (2) tillage versus no-tillage systems, (3) irrigation vs. dryland farming, and (4) herbicide programs (preemergence and/or postemergence) based on the growing seasons from 2017 to 2022. Surveys were distributed via paper copies directly to growers or by email with extension agents assisting in distribution. Of the 49 surveys distributed, 29 were completed and returned, providing a representative sample across counties. The responses covered 29 wheat fields, totaling 77 winter wheat years and 63 summer fallow years, calculated by summing the number of years listed on the surveys. These data were used to calculate the percentages for responses related to crop rotation, tillage practices, irrigation, and herbicide programs.
The most common cropping system in the region was a winter wheat–summer fallow rotation (76%), followed by a winter wheat–spring wheat–summer fallow rotation (14%), and a winter wheat–spring pea rotation (10%). The majority of fields were managed under no-tillage systems (73%) while a smaller portion used conventional tillage (27%). All fields were part of dryland farming (rainfed agriculture). Growers primarily relied on postemergence herbicides (88%) for controlling downy brome in winter wheat with the most commonly used being the ALS inhibitors (Group 2) Beyond (28%) and PowerFlex HL (25%), followed by the PSII inhibitor Tricor DF (Group 5) (16%). Preemergence herbicides were used less frequently (12%) with Zidua (pyroxasulfone; Group 15) (9%) being the most common, followed by Axiom DF (metribuzin + flufenacet; Groups 5 and 15) (3%). In summer fallow fields, RoundUp (glyphosate; Group 9) (89%) was the most commonly used herbicide, followed by Gramoxone (paraquat; Group 22) (11%) for controlling downy brome. No preemergence herbicides were reported for downy brome control in summer fallow.
Herbicide resistance screenings: postemergence and preemergence
A total of 49 downy brome populations were collected from wheat fields of surveyed growers with some growers having multiple fields. A known herbicide-susceptible downy brome population was collected from a non-agricultural area near Adams, OR and included in the resistance screenings for a side-by-side comparison. Herbicides from four distinct modes of action were tested (Table 1), based on their relevance to the winter wheat–summer fallow rotation system and their use in rotational crops. The herbicides tested included ALS inhibitors (Group 2) from different chemical families: Beyond (imidazolinone), Osprey (sulfonylurea), Outrider (sulfonylurea), Olympus (triazolinone), and PowerFlex HL (triazolopyrimidine). Additionally, ACCase inhibitors (Group 1), Select MAX (clethodim) and Assure II (quizalofop), were tested. Select MAX is not registered for use in winter wheat, but it is used in rotational crops such as lentils and fallow. Quizalofop is registered for use in CoAXium wheat only with Aggressor herbicide but not Assure II. Other herbicides tested included Gly Star Original (Group 9), which is registered for use in fallow, and Zidua SC (Group 15), a preemergence herbicide.
| Rate (oz/ac)b | |||||
Herbicide | Active ingredient | Timing | MOA (no.)a | Chemical family | 1× | 2× |
Select MAXc | clethodim | POST | ACCase (1) | cyclohexanedione | 16 | 32 |
Assure IIc | quizalofop | POST | ACCase (1) | aryloxyphenoxy propionate | 12 | 24 |
Beyondd | imazamox | POST | ALS (2) | imidazolinone | 4 | 8 |
Ospreyd | mesosulfuron | POST | ALS (2) | sulfonylurea | 4.75 | 9.5 |
Olympusd | propoxycarbazone | POST | ALS (2) | triazolinone | 0.9 | 1.8 |
PowerFlex HLd | pyroxsulam | POST | ALS (2) | triazolopyrimidine | 2 | 4 |
Outriderd | sulfosulfuron | POST | ALS (2) | sulfonylurea | 0.66 | 1.32 |
TriCor DFd | metribuzin | POST | PSII (5) | triazinone | 8 | 16 |
Gly Star Original | glyphosate | POST | EPSPS (9) | organophosphorus | 24 | 48 |
Zidua SC | pyroxasulfone | PRE | VLCFA (15) | isoxazoline | 3.25 | 6.5 |
aACCase, acetyl-coenzyme A carboxylase; ALS, acetolactate synthase; EPSPS; 5-enolpyruvylshikimate-3-phosphate; PSII, photosystem II; VLCFA, very-long-chain fatty acid. b Rates: 1×, label rate; 2×, two times the label rate. Clethodim and quizalofop label rates were based on the recommendation for lentils, and the glyphosate label rate was based on the recommendation for summer fallow. c Crop oil concentrate (1% v/v) was added to the spray solution. d Nonionic surfactant (0.25% v/v) + ammonium sulfate (2% v/v) was added to the spray solution. | ||||||
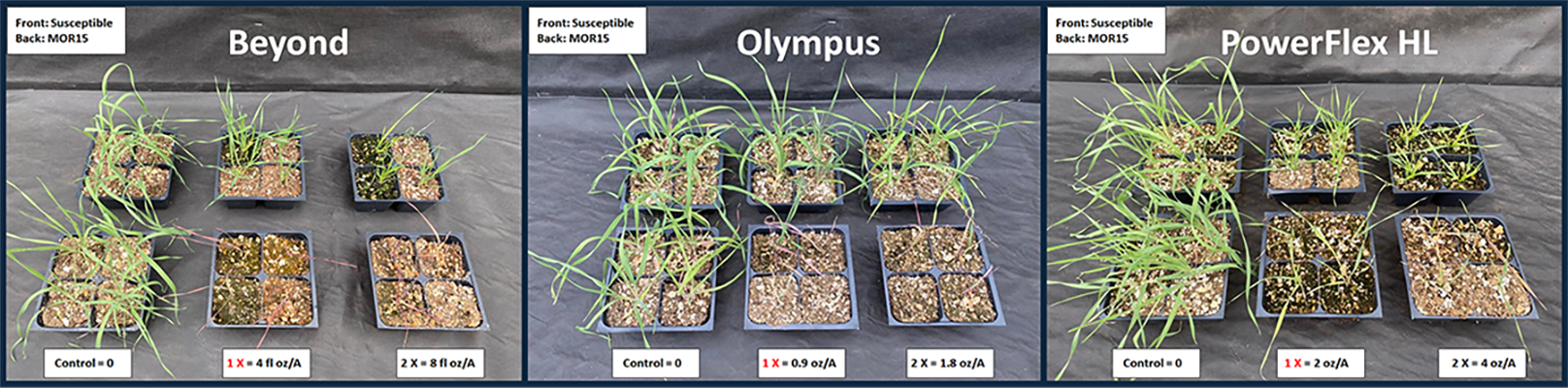
Herbicides were applied at 0, 1, and 2 times (×) the recommended labeled rate (Table 1). For the postemergence screenings, herbicides were applied to downy brome plants at the two- to three-leaf stage. Downy brome plants were visually assessed as dead (completely necrotic plants = 0) or alive (green tissue and evidence of regrowth = 1) 21 days after treatment (DAT). Populations with ≥ 50% survival to the 1× rate of postemergence herbicides were considered resistant. For preemergence screening, the 49 downy brome populations were tested with Zidua at 0, 1×, and 2× rates. Downy brome seedling emergence was counted at 21 DAT.
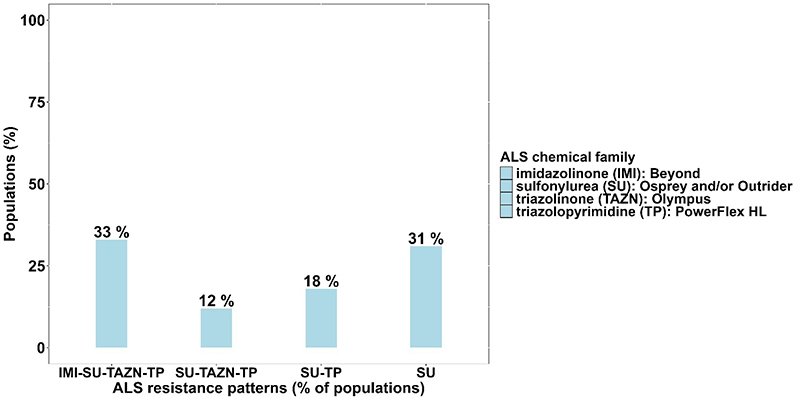
Of the 49 downy brome populations tested, 94% were resistant to at least one ALS inhibitor. Resistance was most prevalent to Osprey and Outrider, occurring in 84% of populations, followed by PowerFlex HL (63%), Olympus (45%), and Beyond (33%) (Figure 1). Among the ALS-resistant populations, 63% exhibited cross-resistance to multiple herbicides within the same mode of action (Figures 2 and 3). For example, 33% of the populations were resistant to herbicides from four chemical families: imidazolinone (Beyond), sulfonylurea (Osprey and/or Outrider), triazolinone (Olympus), and triazolopyrimidine (PowerFlex HL). This indicates a more complex form of resistance, making these populations more difficult to control with a broad spectrum of ALS herbicides. Additionally, 12% exhibited cross-resistance to three of these chemical families while 18% were resistant to both sulfonylurea (Osprey and/or Outrider) and triazolopyrimidine (PowerFlex HL) herbicides. In contrast, 31% were only resistant to sulfonylurea herbicides (Osprey and/or Outrider).
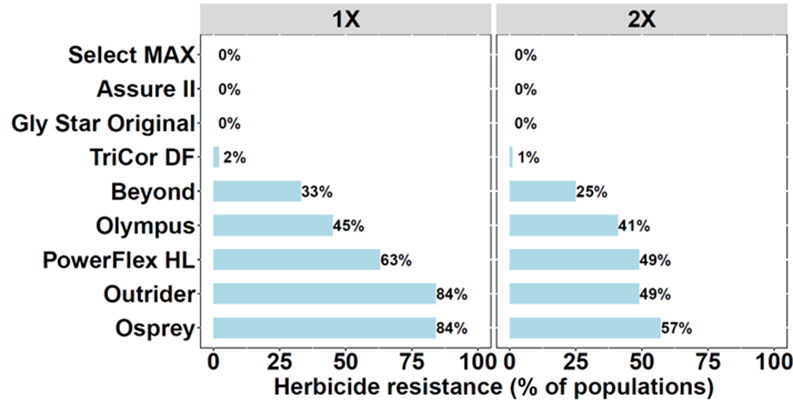
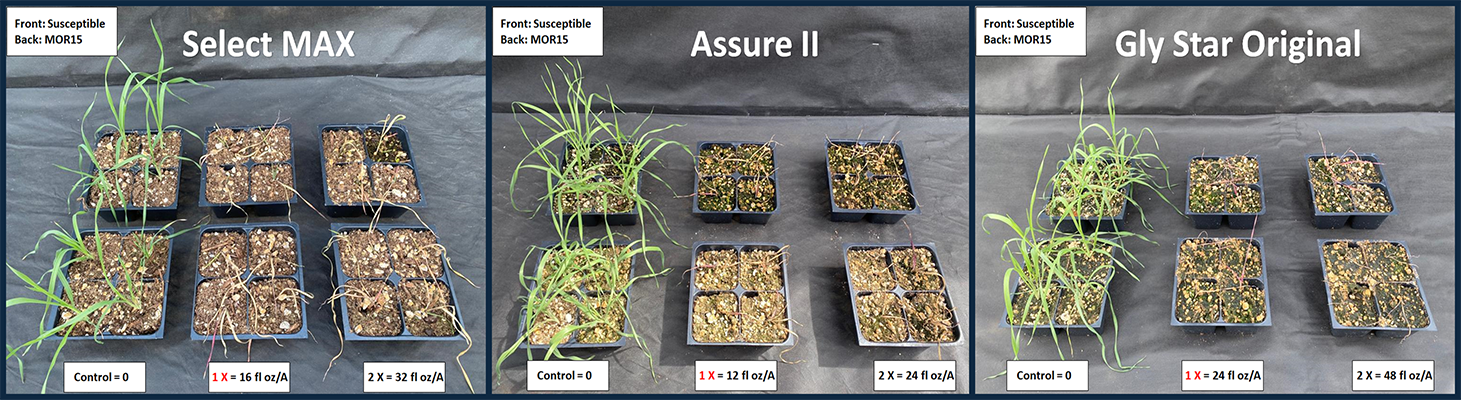
Resistance to the PSII inhibitor TriCor DF was less prevalent, occurring in 2% of populations (Figure 1). These PSII-resistant populations also exhibited multiple resistance to ALS inhibitors (Figure4), meaning they were resistant to herbicides from more than one mode of action. All downy brome populations were susceptible to the postemergence herbicides Select MAX, Assure II, and Gly Star Original (Figure 5) as well as the preemergence herbicide Zidua SC. Surprisingly, no resistance to ACCase inhibitors (Group 1) was detected, despite the historical use of herbicides such as diclofop and tralkoxydim in wheat cropping systems. ACCase-resistant downy brome populations have been reported in other regions of Oregon (Ball et al., 2007; Ribeiro et al., 2023). Similarly, glyphosate resistance in downy brome has already been identified in similar wheat-cropping systems in the neighboring state of Washington (Zuger & Burke, 2020). Therefore, to preserve glyphosate efficacy, growers should use alternative herbicide modes of action or tank mixes in summer fallow.

Key takeaways
Wheat fields across northeastern Oregon contain downy brome populations resistant to both ALS- and PSII-inhibiting herbicides, which are the most commonly used modes of action for controlling this species in winter wheat. The widespread occurrence of cross- and multiple-resistant populations to these herbicides limits the effectiveness of selective herbicide options for downy brome control in wheat. The adoption of quizalofop-resistant wheat (CoAXium) technology provides growers with an additional mode of action (Group 1) and an effective control option for ALS- and PSII-resistant downy brome. Additionally, incorporating preemergence herbicides like Zidua (Group 15) or Anthem Flex (Groups 15 and 14) into downy brome management programs in winter wheat, where low precipitation does not limit herbicide activation, adds more modes of action to the system. This strategy helps delay resistance to current postemergence herbicides and control resistant populations. Future research should focus on integrating nonchemical tactics, such as crop rotation, cover crops, and/or harvest seed weed control technologies (e.g., chaff lining and seed destructors), to manage downy brome seed banks in the dryland wheat-cropping systems of northeastern Oregon.
References
Ball, D.A., Frost, S.M., & Bennett, L.H. (2007). ACCase inhibitor herbicide resistance in downy brome (Bromus tectorum) in Oregon. Weed Science, 55, 91–94.
Hulbert, L.C. (1955). Ecological studies of Bromus tectorum and other annual bromegrasses. Ecological Monographs, 25, 181–213.
Kao, R.H., Brown, C.S., & Hufbauer, R.A. (2008). High phenotypic and molecular variation in downy brome (Bromus tectorum). Invasive Plant Science and Management, 1, 216–225.
Ribeiro, V.H.V., Brunharo, C.A.C.G., Mallory-Smith, C., Walenta, D.L., & Barroso, J. (2023). First report of target-site resistance to ACCase-inhibiting herbicides in Bromus tectorum L. Pest Management Science, 79, 4025–4033.
Rydrych, D.J., & Muzik, T.J. (1968). Downy brome competition and control in dryland wheat. Agronomy Journal, 60, 279–280.
San Martín, C., Thorne, M.E., Gourlie, J.A., Lyon, D.J., & Barroso, J. (2021). Seed retention of grass weeds at wheat harvest in the Pacific Northwest. Weed Science, 69, 238–246.
Zuger, R.J., & Burke, I.C. (2020). Testing in Washington identifies widespread postemergence herbicide resistance in annual grasses. Crops & Soils, 53, 13–18.
Self-Study CEU Quiz
Earn 0.5 CEUs in Integrated Pest Management by taking the quiz for the article at https://web.sciencesocieties.org/Learning-Center/Courses. For your convenience, the quiz is printed below. The CEU can be purchased individually, or you can access as part of your Online Classroom Subscription.
Downy brome is native to the Pacific Northwest and is now a globally distributed weed.
True.
False.
Downy brome can produce up to ___ seeds per plant.
1,200
1,350
1,950
2,000
Of the ___downy brome populations tested in the study, ___ % were resistant to at least one ALS inhibitor.
49, 94
49, 84
49, 76
29, 76
In which of the following herbicides is ALS inhibition the mode of action?
Gly Star Original.
TriCor DF.
Osprey.
Assure II.
In Figure 1, which herbicide(s) were more than 75% of downy brome populations resistant to when tested at 1× the labeled rate?
Olympus.
Olympus and Osprey.
PowerFlex and Olympus.
Outrider and Osprey.
Text © . The authors. CC BY-NC-ND 4.0. Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.