Nitrogen cycle: Unraveling microbial dynamics for optimizing nitrogen use

Nitrogen is a primary macronutrient essential for crop growth, yield, and productivity. To manage nitrogen effectively, it is critical to understand the fate of nitrogen in the soil and the various transformations it undergoes in the nitrogen cycle. Microbes play a vital role in nitrogen cycling by facilitating key processes such as nitrogen fixation, nitrification, and denitrification, which regulate nitrogen availability for plants and nitrogen loss. This article explores the various nitrogen-cycling processes, the microbes involved, and how they function under different environmental conditions. Understanding these microbes and their interactions with environmental factors is essential in improving nitrogen availability and minimizing the environmental impact of nitrogen cycling. Earn 1.5 CEUs in Nutrient Management by taking the quiz for the article.
Nitrogen: Fundamental nutrients for crop production
Nitrogen is a primary macronutrient essential for crop growth and production, serving as a critical component of amino acids, proteins, chlorophylls, and nucleic acids (Zhang et al., 2020). Nitrogen availability regulates plant growth, leaf area development, and photosynthetic performance, directly contributing to crop biomass accumulation and yield formation (Lawlor et al., 2001), thus making nitrogen one of the limiting factors for crop growth, yield, and productivity.

The development of the Haber-Bosch process enabled the production of various forms of reactive nitrogen as a chemical fertilizer, which revolutionized agriculture by meeting the crop nitrogen demands and boosting productivity. The application of nitrogen fertilizers, combined with the positive crop response, played a crucial role in driving the Green Revolution, which significantly increased yields, especially in staple crops like wheat, rice, and corn. This helped to meet the global food demand and allowed countries to achieve food sufficiency and independence (Pingali, 2012). Consequently, between 1960 and 2000, crop yields in many developing countries rose significantly by 208% for wheat, 109% for rice, 157% for corn, 78% for potatoes, and 36% for cassava (Pingali, 2012).
Impacts of excessive nitrogen use on ecosystem
The Green Revolution in the 20th century significantly increased food production and reduced hunger and poverty in many countries, but it also led to the widespread use of synthetic fertilizers, especially nitrogen fertilizers, which contributed to environmental issues like nitrogen leaching into water bodies and greenhouse gas emissions (Pearce, 2018). For example, global nitrogen use increased from 6.6 lb per person in 1960 to 33.0 lb per person in 2018 with North America using 88.2 lb per person (Menegat et al., 2022). In the U.S., nitrogen use in crop production jumped from 0.22 g/m2 per year in 1940 to 9.04 g/m2 per year in 2015—over a 40-fold increase (Cao et al., 2018). This excessive use of nitrogen fertilizers led to a series of environmental issues, such as greenhouse gas emissions, leaching to water bodies, and eutrophication—dense growth of plants in water bodies due to excessive nutrients, causing death of aquatic animals and increasing oxygen demand (Quemada et al., 2013). For instance, nitrate from fertilizers leaches at 26.8 lb/ac per year (Goulding et al., 2000), and nitrous oxide emissions have doubled in the past 60 years (Erisman et al., 2011). More than 90% of rivers in 14 U.S. ecoregions have exceeded safe levels of nitrogen and phosphorus, mainly due to agricultural runoff (Dodds et al., 2009).
While enough nitrogen fertilizers are being produced to feed the world, the pressing challenges are improving nitrogen use efficiency and reducing environmental depositions and greenhouse gas emissions. To reduce the environmental impacts, the International Nitrogen Management System, an initiative by the United Nations Environment Program, has set a goal to cut down nitrogen waste to half by 2030, emphasizing a holistic approach to nitrogen management (INMS, 2018). Thus, for effective nitrogen management, it is critical to understand the fate of nitrogen in the soil and the various transformations it undergoes in the nitrogen cycle.
Nitrogen cycle
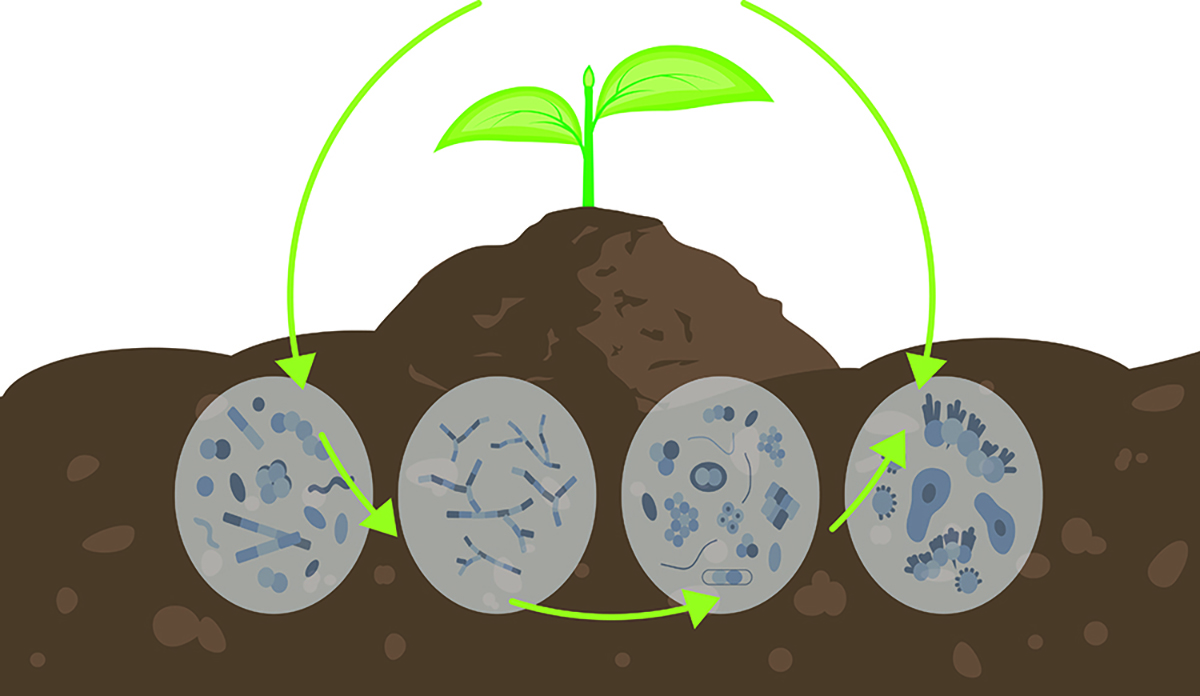
The nitrogen cycle is a fundamental biogeochemical process that describes the movement of nitrogen through the Earth's atmosphere, biosphere, lithosphere, and hydrosphere and the transformation of nitrogen from one form to another. In agriculture, it is critical as it underpins nitrogen availability, a key nutrient essential for crop growth. Improving our understanding of the nitrogen cycle will help us to efficiently utilize available soil nitrogen and plan for external nitrogen inputs with minimal cost and environmental concerns.
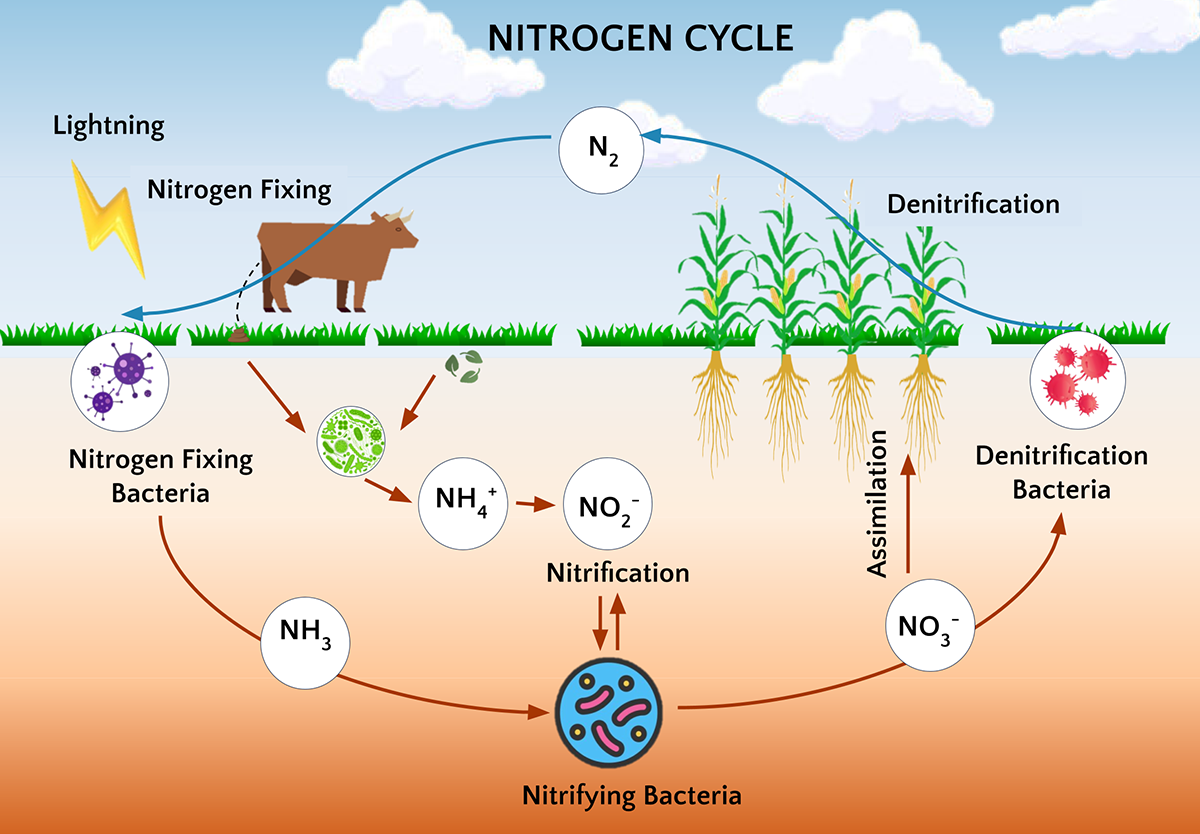
The nitrogen cycle can be divided into three processes: decomposition, assimilation, and dissimilation (Levy-Booth et al., 2014), all of which involve soil microbes that control the rate and extent of nitrogen transformations in the soil (Kuypers et al., 2018). The decomposition process, also known as nitrogen mineralization, involves soil microbes breaking down soil organic matter, such as plant residues and dead microbes, and releasing ammonia or inorganic ammonium (NH₄⁺) ions. The assimilation process involves incorporating soil inorganic nitrogen such as ammonium, nitrate, or nitrite ions into microbial or plant biomass. The dissimilation process refers to the reduction or oxidation of nitrogen compounds and is primarily used in microbial respiration to generate energy without being incorporated into biomass.
The dissimilation process includes conversions of nitrogen between various forms during microbial metabolism. For example, nitrogen is fixed from atmospheric nitrogen to ammonium form, which can then be oxidized to nitrate through nitrification. Nitrate can be reduced to nitrous oxide and atmospheric nitrogen through denitrification. Under certain conditions, nitrate may also be reduced to ammonium through dissimilatory nitrate reduction to the ammonium process (Mohan & Cole, 2007) or the anaerobic ammonia oxidation process (Kuenen, 2008).
These transformations, driven by energy released during oxidation or reduction, play a critical role in nitrogen cycling but do not result in nitrogen being directly incorporated into the biomass of plants or microbes.
Microbes play a vital role in nitrogen cycling by facilitating key processes such as nitrogen fixation, nitrification, and denitrification, which regulate nitrogen availability for plants and nitrogen loss. The activity and composition of microbial communities determine the rate and fate of nitrogen cycling. At the same time, environmental factors such as precipitation, drought, pH, salinity, and organic matter significantly impact microbial functions. The following sections will explore the various nitrogen-cycling processes, the microbes involved, and how they function under different environmental conditions. Understanding these microbes and their interactions with environmental factors is essential in improving nitrogen availability and minimizing the environmental impact of nitrogen cycling.
Nitrogen-fixing microbes
Atmospheric nitrogen represents the largest nitrogen pool, but it is unavailable for direct uptake by plants. Unlike carbon, plants cannot solely fix nitrogen from the atmosphere. The primary entry of nitrogen into terrestrial ecosystems occurs through microbial fixation where nitrogen-fixing microbes convert atmospheric nitrogen into a bioavailable ammonium form (Wheatcroft & Watson, 1988). This process is catalyzed by a group of nitrogenase enzymes (Kuypers et al., 2018). Nitrogen-fixing microbes include free-living bacteria such as Azotobacter, Azospirillum, and Clostridium, which do not require plants as a host (Soumare et al., 2020), as well as symbiotic bacteria such as Rhizobium, which form nodules on the legume roots and fix nitrogen in exchange for carbon (Ravikumar et al., 2007). Further, some bacteria-like-unicellular organisms, known as archaea, such as Methanobacteriales, Methanococcales, and Methanosarcinales, are also reported to fix atmospheric nitrogen in soil and water bodies.

The discovery of nitrogen-fixing microorganisms has led to the development of numerous commercial biofertilizers worldwide (Bhattacharjee et al., 2008; Mahanty et al., 2017; Soumare et al., 2020). Although synthetic fertilizers currently support 48% of the global population (Ritchie, 2017), their widespread use has several environmental consequences associated with inorganic fertilizers, such as eutrophication and greenhouse gas emissions (Pahalvi et al., 2021). Crop rotation with leguminous species can enhance natural nitrogen storage in the soil, boosting crop yields and improving soil fertility ( Rosolem et al., 2017; Ye et al., 2021; Ghimire et al., 2024).
Nitrogen fixation is joint in legumes but less common in cereal crops. However, recent research has shown that nitrogen fixation can occur in cereal crops and contribute to crop nitrogen demand. Free-living nitrogen-fixing bacteria contribute approximately 20–25% of the nitrogen requirements for crops like rice and corn (Montañez et al., 2012). These bacteria can be found in the rhizosphere or plant organs and can fix nitrogen in hypoxic or aerobic conditions (Thaweenut et al., 2011).

For instance, Bradyrhizobium can fix nitrogen in legumes and non-legumes under hypoxic conditions. Some methanotrophs, such as Methylosinus and Methylocystis, can also fix atmospheric nitrogen in rice roots by colonizing intercellular spaces (Yoneyama et al., 2019). Recently, a corn variety from Mexico, Sierra Mixe, was discovered to produce hypoxic mucilage around its aerobic roots, containing nitrogen-fixing bacteria that can fix nitrogen and supply up to 29–82% of the plant’s nitrogen requirements (Bennett et al., 2020). Similar mucilage has also been detected in small amounts in other common cereal crops like wheat, barley, and sorghum, suggesting that these crops may use this strategy to meet their nitrogen needs (Bennett et al., 2020). Further, applying free-living nitrogen-fixing bacteria in cereal crops such as wheat, rice, and corn can help reduce reliance on synthetic nitrogen fertilizers.
Decomposition (nitrogen mineralization) microbes
Most forms of organic nitrogen in soil are unavailable to plants due to their complex chemical structures, thus requiring microbial-mediated decomposition. Microbial decomposition or nitrogen mineralization occurs when microorganisms convert organic nitrogen from various organic substrates into a bioavailable ammonium form (NH4+) (Kitchen & Goulding, 2001; Séneca et al., 2021). The nitrogen mineralization process involves two key stages: aminization and ammonification. Aminization is a process where complex proteins are broken into simpler amino acids, amides, and amines, facilitated by extracellular enzymes secreted by heterotrophic bacteria and fungi (Schimel & Bennett, 2004; Myrold, 2021). Ammonification is a process where simpler nitrogen molecules like amino acids, amides, and amines, generated from aminization, are converted into ammonium ions, facilitated by autotrophic bacteria such as Nitrosomonas and Nitrobacter (Mohanty et al., 2013).
Nitrogen mineralization is tightly coupled with immobilization where soil microbes assimilate ammonium ions (NH4+) into biomass. The carbon-to-nitrogen (CN) ratio of organic matter in soil plays a crucial role in determining whether mineralization or immobilization occurs. This ratio can vary widely, from 6.5 to 664, depending on the source and age of organic amendments (Aulakh et al., 1991; Huang et al., 2004). For example, an organic compound with a CN ratio of 15 means that one unit of nitrogen is made available for every 15 units of carbon released during decomposition. Therefore, the lower CN ratio is associated with higher nitrogen mineralization, indicating a negative correlation between nitrogen mineralization and the CN ratio (Ye et al., 2018).
In general, immobilization occurs when the CN ratio is higher than 24, and mineralization occurs when the CN ratio is lower than 24 (Reddy et al., 2022). Thus, during agricultural practice, applying high CN ratio organic materials and adopting suitable management practices to increase soil organic matter content decreases nitrogen mineralization, promoting the slow release of nitrogen sources to the plants (Ghimire et al., 2024; Gannett et al., 2024). This reverse fertilization method, limiting nitrogen mineralization by increasing the CN ratio, can be an alternative effective weed management tool in agroecosystems (Gannett et al., 2024).
Nitrification microbes
Nitrification is a process of oxidation of ammonium ions into nitrite and nitrate ions in two-step reactions, nitrite being the product of the first-step reaction and nitrate being the product of the second-step reaction, serving as the rate-limiting step in the nitrogen cycle. This process is carried out by two groups of gram-negative microorganisms. In the first step, ammonium (NH₄⁺) is converted to nitrite (NO₂⁻) by ammonia-oxidizing archaea, such as Nitrososphaera, and ammonia-oxidizing bacteria, such as Nitrosomonas, Nitrosococcus, and Nitrosospira spp. The second step involves the conversion of nitrite (NO₂⁻) to nitrate (NO₃⁻), which is carried out by nitrite-oxidizing bacteria, such as Nitrobacter spp. Additionally, recent studies have identified complete ammonia-oxidizing bacteria that can convert ammonium ions directly to nitrate ions.
Nitrification in soil regulates the nitrogen pool, its availability and loss, determining the fate and efficiency of nitrogen inputs. Further, land management heavily determines the nitrification activity (Jordan et al., 1979; Cassman et al., 2002). For example, in undisturbed soil ecosystems, less than 10% of total nitrogen undergoes nitrification (Haynes et al., 1986). In contrast, more than 95% of total nitrogen flows through the nitrification–denitrification pathway in modern agricultural systems (highly disturbed soil ecosystems) (Subbarao et al., 2013). Agricultural ecosystems typically have open nitrogen cycles where various nitrogen forms can be lost without going into the cycling process, resulting in low nitrogen recovery and efficiency (Cassman et al., 2002; Dinnes et al., 2020). Furthermore, the separation of livestock from crop production has disrupted nutrient cycling, depleted soil organic matter, altered soil physical and chemical properties, and shifted soil microbial activity. This has created high-nitrifying soil environments, causing more ammonium forms to convert into nitrate forms, that are prone to leaching and emissions (Tilman et al., 2002).

The various soil and environmental factors can be utilized to manage nitrification microbes and nitrogen pools. While increasing nitrification microbes can alleviate soil nitrogen limitations in the long term, excessive nitrification can also increase nitrate ions, which are more prone to leaching. The conversion of ammonium ions to nitrate ions also increases the risk of nitrogen loss from leaching or atmospheric emissions through denitrification. To mitigate these losses, plants have evolved mechanisms to limit nitrification. For example, some plants produce and release nitrification-inhibiting compounds from their roots, known as biological nitrification inhibitors (BNIs), which inhibit the nitrification process (Subbarao et al., 2007). Thus, BNIs are crucial for improving nitrogen use efficiency, particularly in low-nitrogen soils. By breeding crops with strong BNI traits, we can achieve sustainable nitrogen management systems that reduce fertilizer use, nitrogen loss, and greenhouse gas emissions. The effectiveness of BNIs is influenced by genetics, soil, and environmental factors. Understanding the genetic basis of BNIs and breeding crops carrying BNI traits can help develop climate-resilient and sustainable nitrogen management systems, reducing nitrogen loss and greenhouse gas emissions.

Denitrification microbes
Denitrification is a critical microbial process in the nitrogen cycle where microbes use oxidized nitrogen compounds (NO₃⁻) as an energy source and produce reduced forms of nitrogen, such as nitrous oxide and nitrogen gas, that are released into the atmosphere. Complete denitrification is a stepwise reduction process carried out by various microbes, which involves the conversion of nitrate into nitrite ions, then to nitric oxide, nitrous oxide, and finally into nitrogen gas (Demanèche et al., 2009; Bru et al., 2011). The major denitrifying bacteria are Bacillus, Enterobacter, Micrococcus, Pseudomonas, Spirillum, Aerobacter, and Flavobacterium spp. This is a major pathway for soil nitrogen loss and has significant ecological impacts due to the release of nitrous oxide, a potent greenhouse gas with a high global-warming potential, predominantly emitted from agricultural activities (World Resources Institute, 2022).
Nitrogen fertilizers, often in the form of ammoniacal nitrate, are susceptible to denitrification, resulting in nitrogen loss through gaseous emissions. Thus, efficient nitrification management is necessary to minimize denitrification losses in agricultural systems. For example, chemical compounds such as nitrapyrin [2-chloro-6-(trichloromethyl)-pyridine] and DMPP (3,4-dimethylpyrazole phosphate) have been shown to reduce nitrification activity and minimize losses through leaching and denitrification (Wendeborn, 2020). Additionally, maintaining crop diversity through cover cropping during fallow periods helps assimilate more nitrogen, reducing leaching, lowering nitrous oxide emissions, and improving overall soil health and nutrient availability (Wang et al., 2024).

Anaerobic conditions also favor denitrification with bacteria and archaea being the major denitrifiers. While denitrification can reduce nitrate leaching into ground and water bodies, mitigating some adverse effects of excessive nitrogen fertilizer use, it also has negative consequences such as nitrous oxide, causing the greenhouse effect, ozone layer depletion, and the loss of nitrogen that could otherwise support plant growth (Munch et al., 2007). It is essential to know that agriculture is responsible for 80% of nitrous oxide emissions with concentrations rising from 270 ppb in 1980 to 319 ppb in 2012 (Haider et al., 2020), highlighting the challenges posed by agriculture to global warming and ozone depletion. Further, nitrous oxide is produced not only by denitrifiers, but also by nitrifiers in some specific conditions. For example, in poor sandy soils with suboptimal moisture levels for denitrification, nitrifying bacteria cause denitrification, leading to nitrous oxide emissions. This demonstrates that nitrous oxide emission pathways are influenced by soil moisture, aeration, and other environmental factors, thus indicating that proper soil and crop management practices can optimize nitrogen uptake and emissions.
Dissimilatory nitrate reduction microbes
In addition to the conversion of nitrate into nitrous oxide, nitrate can also be reduced to ammonium through a process known as dissimilatory nitrate reduction to ammonia (DNRA), which allows nitrogen to remain sequestered within the soil rather than escaping into the atmosphere. For example, some microbes use nitrate ions as an electron acceptor to perform anaerobic respiration and yield ammonium ions (Ambus & Zechmeister-Boltenstern, 2007). These microbes can be broadly divided into two groups based on their energy conservation mode. The first group, which includes the gram-negative bacteria Desulfovibrio and Wolinella spp., conducts respiratory DNRA in the periplasm, a region between the cell wall and the cell membrane. The second group, which includes the gram-negative bacteria Escherichia coli and Klebsiella spp., performs fermentative DNRA in the cytoplasm and utilizes organic matter to reduce nitrate into ammonium ions (Pandey et al., 2020).
Low nitrate conditions with a high CN ratio generally favor DNRA over denitrification (Pandey et al., 2020). Further, the occurrence and efficiency of the DNRA process are influenced by several factors, including soil and sediment oxidation states, CN ratio, concentration ratio of nitrite to nitrate, and concentrations of iron (Fe²⁺) and sulfide (S²⁻) ions (Putz et al., 2018). For example, a high CN ratio favors DNRA under low-redox conditions while a low ratio promotes denitrification. If the CN ratio is equal, the nitrite-to-nitrate ratio influences nitrate partitioning with a higher ratio favoring DNRA. Additionally, a high sulfide-to-nitrate ratio promotes DNRA in coastal ecosystems and saline sediments, and a higher concentration of iron levels enhances DNRA in river systems. However, its impact on agricultural land remains uncertain. These microbes thrive in soils with abundant organic matter and soil moisture. For example, DNRA microbes account for 99% of nitrate consumption in forest soils and 21% in rice fields (Yin et al., 2002; Templer et al., 2008). Thus, DNRA microbes play an essential role in terrestrial ecosystems by reducing nitrate leaching, enriching plant-available ammonium, and reducing nitrogen gas emissions.
Anaerobic ammonium oxidation microbes
Some bacteria metabolically combine ammonium and nitrate or nitrite ions to form nitrogen gas (Kuenen, 2008). These bacteria are also known as anaerobic ammonium oxidation (anammox) bacteria. In this process, nitrite, nitrate, and ammonium serve as electron acceptors and donors, respectively, and the reaction yields nitrogen gas. Anammox microbes are anoxic and lithotrophic bacteria categorized in the Planctomycetales order (van Niftrik & Jetten, 2012). Anammox microbes were first found in aquatic environments but later identified in terrestrial ecosystems such as grasslands and agricultural soils (Schubert et al., 2006; Schmid et al., 2007). Interestingly, some anammox bacteria can switch to a heterotrophic lifestyle, utilizing organic compounds such as formate and acetate to generate biomass. However, this process is energy intensive—fixing one mole of carbon into biomass requires 15 cycles of ammonium oxidation, which leads to a slow growth rate for these microbes (Kartal et al., 2013).
Although anammox bacteria represent only a tiny fraction of the nitrogen-cycling microbial community, their critical role in ecosystems is significant. They can also be applied in wastewater treatment plants for ammonium removal (Chávez-Crooker & Obreque-Contreras, 2010). Thus, enhancing the productivity and efficiency of anammox bacteria could bring significant economic and environmental benefits, particularly in nitrogen management in agricultural ecosystems.
Optimizing the nitrogen cycle for better nitrogen management
Nitrogen cycling is critical in agricultural systems by influencing soil fertility, crop productivity, and environmental sustainability. The key processes, such as nitrification, denitrification and nitrogen-fixing, along with nitrogen mineralization, regulate the availability of plant-usable nitrogen while affecting greenhouse gas emissions, particularly nitrous oxide. Environmental factors such as drought, salinity, pH, and agronomic practices regulate the soil nitrogen cycles. For instance, drought conditions can increase nitrogen loss through nitrogen gas emissions due to higher concentrations of nitrates in drought-prone soil (Sher et al., 2012). Drought also strongly affects nitrifier microbial activity, where the activity of ammonia-oxidizing bacteria increases and the activity of ammonia-oxidizing archaea decreases, indicating that archaea are more sensitive to drought and, thus, need proper consideration in management practices (Seneca et al., 2020). Further, abundance and activity of denitrifiers are regulated by soil chemical and physiological properties, such as pH and temperature increase (Heil et al., 2015). This indicates that land management, agroecosystem, and cropping systems significantly influence nitrifying and denitrifying activity and nutrient availability.

Further, some bacteria, such as Azorhizobium, can be both nitrogen-fixing and denitrifying and offer a potential for enhancing nitrogen fixation and its availability in agricultural soils. Fertilizer application practices also play a significant role in the dynamics of nitrogen-cycling microbial communities. For example, the higher dosage of nitrogen application can stimulate denitrification, leading to nitrous oxide emission while slow-release nitrogen fertilizers help mitigate this effect (Yang et al., 2009). Practices like green manure application, direct seeding, and mulching with crop residues can also increase denitrification activity and nitrous oxide emissions due to a higher CN ratio in the organic matter (Baudoin et al., 2009). Therefore, adopting site-specific, sustainable management practices such as optimizing nitrogen application and reducing nitrogen leaching can balance the nitrogen cycle, improve soil health, and mitigate environmental impacts.
Future perspective: Sustainable nitrogen management
In the face of climate change, the different climatic and edaphic factors influence the stability and fluctuations of nitrogen cycling. Proper field and controlled environment studies should be conducted to comprehensively understand climate change impacts on the nitrogen cycle in the global and agricultural context. Sustainable agricultural management practices like crop rotation, fertilizers, and bio-amendment applications based on the strength and vulnerability of each nitrogen-cycling step in a particular agricultural land can be adopted to maximize the nitrogen usage and minimize the nitrogenous gas emission and leaching.
Given the potential of nitrogen-cycling microbes to regulate various soil nitrogen pools, future research could focus on how sustainable management, such as reduced tillage and cover cropping, could promote the growth of nitrogen-cycling microbial communities, control nitrogen leaching, and reduce the dependence on synthetic nitrogen fertilizers. Further, breeding high-nitrogen-use efficiency crops with traits such as BNIs and symbiosis with nitrogen-fixing bacteria can help maximize nitrogen use and reduce the pressure on nitrogen application in agricultural practices. The global scale estimation of nitrogen deposition into the soil and water ecosystems across different biomes should be conducted to formulate the policy on nitrogen application and management strategies for sustainable soil health and crop productivity, enhancing balanced nitrogen cycles. Government-level interventions through incentives and subsidies are essential in changing nitrogen use and deposition landscapes.
Ambus, P., & Zechmeister-Boltenstern, S. (2007). Denitrification and N-cycling in forest ecosystems. In Biology of the nitrogen cycle (pp. 343–358). Elsevier. https://doi.org/10.1016/B978-044452857-5.50023-0
Aulakh, M. S., Walters, D. T., Doran, J. W., Francis, D. D., & Mosier, A. R. (1991). Crop residue type and placement effects on denitrification and mineralization. Soil Science Society of America Journal, 55(4), 1020–1025. https://doi.org/10.2136/sssaj1991.03615995005500040022x
Bennett, A. B., Pankievicz, V. C., & Ané, J. M. (2020). A model for nitrogen fixation in cereal crops. Trends in plant science, 25(3), 226–235.
Bhattacharjee, R. B., Singh, A., & Mukhopadhyay, S. N. (2008). Use of nitrogen-fixing bacteria as biofertilizer for non-legumes: prospects and challenges. Applied Microbiology and Biotechnology, 80(2), 199–209. https://doi.org/10.1007/s00253-008-1567-2
Cao, P., Lu, C., & Yu, Z. (2018). Historical nitrogen fertilizer use in agricultural ecosystems of the contiguous United States during 1850–2015: application rate, timing, and fertilizer types. Earth System Science Data, 10(2), 969–984.
Cassman, K. G., Dobermann, A., & Walters, D. T. (2002). Agroecosystems, nitrogen-use efficiency, and nitrogen management. AMBIO: A Journal of the Human Environment, 31(2), 132–140.
Chávez-Crooker, P., & Obreque-Contreras, J. (2010). Bioremediation of aquaculture wastes. In Current Opinion in Biotechnology, 21(3), 313–317. https://doi.org/10.1016/j.copbio.2010.04.001
Demanèche, S., Philippot, L., David, M. M., Navarro, E., Vogel, T. M., & Simonet, P. (2009). Characterization of denitrification gene clusters of soil bacteria via a metagenomic approach. Applied and Environmental Microbiology, 75(2), 534–537. https://doi.org/10.1128/AEM.01706-08
Dinnes, D. L., Karlen, D. L., Jaynes, D. B., Kaspar, T. C., Hatfield, J. L., Colvin, T. S., & Cambardella, C. A. (2002). Nitrogen management strategies to reduce nitrate leaching in tile‐drained Midwestern soils. Agronomy Journal, 94(1), 153–171.
Dodds, W. K., Bouska, W. W., Eitzmann, J. L., Pilger, T. J., Pitts, K. L., Riley, A. J., Schloesser, J. T., & Thornbrugh, D. J. (2009). Eutrophication of US freshwaters: analysis of potential economic damages. ACS Publications.
Erisman, J. W., Galloway, J., Seitzinger, S., Bleeker, A., & Butterbach-Bahl, K. (2011). Reactive nitrogen in the environment and its effect on climate change. Current Opinion in Environmental Sustainability, 3(5), 281–290.
Gannett, M., DiTommaso, A., Sparks, J. P., & Kao-Kniffin, J. (2024). Microbial nitrogen immobilization as a tool to manage weeds in agroecosystems. Agriculture, Ecosystems & Environment, 366, 108904. https://doi.org/10.1016/j.agee.2024.108904
Ghimire, O. P., Lazo, A., Parajuli, B., & Nepal, J. (2024). Fostering microbial activity and diversity in agricultural systems: adopting better management practices and strategies: Part 2. CSA News. https://doi.org/10.1002/csan.21344
Goulding, K. W. T., Poulton, P. R., Webster, C. P., & Howe, M. T. (2000). Nitrate leaching from the Broadbalk Wheat Experiment, Rothamsted, UK, as influenced by fertilizer and manure inputs and the weather. Soil Use and Management, 16(4), 244–250. https://doi.org/10.1111/j.1475-2743.2000.tb00203.x
Haider, A., Bashir, A., & ul Husnain, M. I. (2020). Impact of agricultural land use and economic growth on nitrous oxide emissions: Evidence from developed and developing countries. Science of the Total Environment, 741, 140421.
Haynes, R. J., & Sherlock, R. R. (1986). Gaseous losses of nitrogen. In Mineral nitrogen in the plant-soil system (pp. 242–302). Academic Press.
Heil, J., Liu, S., Vereecken, H., & Brueggemann, N. (2015). Abiotic nitrous oxide production from hydroxylamine in soils and their dependence on soil properties. Soil Biology and Biochemistry, 84, 107–115. https://doi.org/10.1016/j.soilbio.2015.02.022
Huang, G. F., Wong, J. W. C., Wu, Q. T., & Nagar, B. B. (2004). Effect of C/N on composting of pig manure with sawdust. Waste Management, 24(8), 805–813. https://doi.org/10.1016/j.wasman.2004.03.011
INMS. (2018). Towards the establishment of an International Nitrogen Management System. http://www.inms.international
Jordan, C. F., Todd, R. L., & Escalante, G. (1979). Nitrogen conservation in a tropical rain forest. Oecologia, 39, 123–128.
Kartal, B., de Almeida, N. M., Maalcke, W. J., Op den Camp, H. J. M., Jetten, M. S. M., & Keltjens, J. T. (2013). How to make a living from anaerobic ammonium oxidation. FEMS Microbiology Reviews, 37(3), 428–461. https://doi.org/10.1111/1574-6976.12014
Kitchen, N. R., & Goulding, K. W. T. (2001). On-farm technologies and practices to improve nitrogen use efficiency. In Nitrogen in the Environment: Sources, problems and management (pp. 335–369). Elsevier. https://doi.org/10.1016/B978-044450486-9/50015-7
Kuenen, J. G. (2008). Anammox bacteria: from discovery to application. Nature Reviews Microbiology, 6(4), 320–326. https://doi.org/10.1038/nrmicro1857
Kuypers, M. M., Marchant, H. K., & Kartal, B. (2018). The microbial nitrogen-cycling network. Nature Reviews Microbiology, 16(5), 263–276.
Lawlor, D. W., Lemaire, G., & Gastal, F. (2001). Nitrogen, plant growth and crop yield. In Plant nitrogen (pp. 343–367). Springer.
Levy-Booth, D. J., Prescott, C. E., & Grayston, S. J. (2014). Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biology and Biochemistry, 75, 11–25. Elsevier. https://doi.org/10.1016/j.soilbio.2014.03.021
Mahanty, T., Bhattacharjee, S., Goswami, M., Bhattacharyya, P., Das, B., Ghosh, A., & Tribedi, P. (2017). Biofertilizers: a potential approach for sustainable agriculture development. Environmental Science and Pollution Research, 24(4), 3315–3335. https://doi.org/10.1007/s11356-016-8104-0
Menegat, S., Ledo, A., & Tirado, R. (2022). Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Scientific Reports, 12(1), 1–13.
Mohan, S. B., & Cole, J. A. (2007). The dissimilatory reduction of nitrate to ammonia by anaerobic bacteria. In Biology of the nitrogen cycle (pp. 93–106). Elsevier. https://doi.org/10.1016/B978-044452857-5.50008-4
Mohanty, M., Sinha, N. K., Sammi Reddy, K., Chaudhary, R. S., Subba Rao, A., Dalal, R. C., & Menzies, N. W. (2013). How important is the quality of organic amendments in relation to mineral N availability in soils? Agricultural Research, 2(2), 99–110. https://doi.org/10.1007/s40003-013-0052-z
Montañez, A., Blanco, A. R., Barlocco, C., Beracochea, M., & Sicardi, M. (2012). Characterization of cultivable putative endophytic plant growth promoting bacteria associated with maize cultivars (Zea mays L.) and their inoculation effects in vitro. Applied Soil Ecology, 58, 21–28.
Munch, J. C., & Velthof, G. L. (2007). Denitrification and agriculture. In Biology of the nitrogen cycle (pp. 331–341). Elsevier.
Myrold, D. D. (2021). Transformations of nitrogen. In Principles and applications of soil microbiology (pp. 385–421). Elsevier. https://doi.org/10.1016/B978-0-12-820202-9.00015-0
Pahalvi, H. N., Rafiya, L., Rashid, S., Nisar, B., & Kamili, A. N. (2021). Chemical fertilizers and their impact on soil health. In Microbiota and biofertilizers, Vol 2 (pp. 1–20). Springer International Publishing. https://doi.org/10.1007/978-3-030-61010-4_1
Pandey, C. B., Kumar, U., Kaviraj, M., Minick, K. J., Mishra, A. K., & Singh, J. S. (2020). DNRA: A short-circuit in biological N-cycling to conserve nitrogen in terrestrial ecosystems. Science of the Total Environment, 738, 139710. Elsevier B.V. https://doi.org/10.1016/j.scitotenv.2020.139710
Pearce, F. (2018). Can the world find solutions to the nitrogen pollution crisis? Yale Environment, 360(6).
Pingali, P. L. (2012). Green revolution: impacts, limits, and the path ahead. Proceedings of the National Academy of Sciences, 109(31), 12302–12308.
Putz, M., Schleusner, P., Rütting, T., & Hallin, S. (2018). Relative abundance of denitrifying and DNRA bacteria and their activity determine nitrogen retention or loss in agricultural soil. Soil Biology and Biochemistry, 123, 97–104. https://doi.org/10.1016/j.soilbio.2018.05.006
Quemada, M., Baranski, M., & Agriculture, M. N. L.-. (2013). Meta-analysis of strategies to control nitrate leaching in irrigated agricultural systems and their effects on crop yield. Agriculture, Ecosystems & Environment, 174(15), 1–10.
Ravikumar, S., Kathiresan, K., Alikhan, S. L., Williams, G. P., Anitha, N., & Gracelin, A. (2007). Growth of Avicennia marina and Ceriops decandra seedlings inoculated with halophilic Azotobacters. Journal of Environmental Biology, 28, 601–603.
Reddy, K. R., DeLaune, R. D., & Inglett, P. W. (2022). Biogeochemistry of wetlands: science and applications. CRC press.
Ritchie, H. (2017). How many people does synthetic fertilizer feed? https://ourworldindata.org/how-many-people-does-synthetic-fertilizer-feed
Rosolem, C. A., Ritz, K., Cantarella, H., Galdos, M. V., Hawkesford, M. J., Whalley, W. R., & Mooney, S. J. (2017). Enhanced plant rooting and crop system management for improved N use efficiency. Advances in Agronomy, 205–239. https://doi.org/10.1016/bs.agron.2017.07.002
Schimel, J. P., & Bennett, J. (2004). Nitrogen mineralization: challenges of a changing paradigm. Ecology, 85(3), 591–602. https://doi.org/10.1890/03-8002
Schmid, M. C., Risgaard‐Petersen, N., Van De Vossenberg, J., Kuypers, M. M. M., Lavik, G., Petersen, J., … & Jetten, M. S. M. (2007). Anaerobic ammonium‐oxidizing bacteria in marine environments: widespread occurrence but low diversity. Environmental Microbiology, 9(6), 1476–1484. https://doi.org/10.1111/j.1462-2920.2007.01266.x
Schubert, C. J., Durisch‐Kaiser, E., Wehrli, B., Thamdrup, B., Lam, P., & Kuypers, M. M. M. (2006). Anaerobic ammonium oxidation in a tropical freshwater system (Lake Tanganyika). Environmental Microbiology, 8(10), 1857–1863. https://doi.org/10.1111/j.1462-2920.2006.01074.x
Séneca, J., Pjevac, P., Canarini, A., Herbold, C. W., Zioutis, C., Dietrich, M., ... & Richter, A. (2020). Composition and activity of nitrifier communities in soil are unresponsive to elevated temperature and CO2, but strongly affected by drought. The ISME Journal, 14(12), 3038–3053.
Séneca, J., Söllinger, A., Herbold, C. W., Pjevac, P., Prommer, J., Verbruggen, E., Sigurdsson, B. D., Peñuelas, J., Janssens, I. A., Urich, T., Tveit, A. T., & Richter, A. (2021). Increased microbial expression of organic nitrogen cycling genes in long-term warmed grassland soils. ISME Communications, 1(1). https://doi.org/10.1038/s43705-021-00073-5
Sher, Y., Zaady, E., Ronen, Z., & Nejidat, A. (2012). Nitrification activity and levels of inorganic nitrogen in soils of a semi-arid ecosystem following a drought-induced shrub death. European journal of soil biology, 53, 86–93.
Soumare, A., Diedhiou, A. G., Thuita, M., Hafidi, M., Ouhdouch, Y., Gopalakrishnan, S., & Kouisni, L. (2020). Exploiting biological nitrogen fixation: A route towards a sustainable agriculture. Plants, 9(8), 1011. https://doi.org/10.3390/plants9081011
Subbarao, G. V., Rondon, M., Ito, O., Ishikawa, T., Rao, I. M., Nakahara, K., ... & Berry, W. L. (2007). Biological nitrification inhibition (BNI)—is it a widespread phenomenon? Plant and Soil, 294, 5–18.
Subbarao, G. V., Sahrawat, K. L., Nakahara, K., Rao, I. M., Ishitani, M., Hash, C. T., ... & Lata, J. C. (2013). A paradigm shift towards low-nitrifying production systems: the role of biological nitrification inhibition (BNI). Annals of Botany, 112(2), 297–316.
Templer, P. H., Silver, W. L., Pett-Ridge, J., M. DeAngelis, K., & Firestone, M. K. (2008). Plant and microbial controls on nitrogen retention and loss in a humid tropical forest. Ecology, 89(11), 3030–3040. https://doi.org/10.1890/07-1631.1
Thaweenut, N., Hachisuka, Y., Ando, S., Yanagisawa, S., & Yoneyama, T. (2011). Two seasons’ study on nifH gene expression and nitrogen fixation by diazotrophic endophytes in sugarcane (Saccharum spp. hybrids): expression of nifH genes similar to those of rhizobia. Plant and soil, 338, 435–449.
Tilman, D., Cassman, K. G., Matson, P. A., Naylor, R., & Polasky, S. (2002). Agricultural sustainability and intensive production practices. Nature, 418(6898), 671–677.
Van Deynze, A., Zamora, P., Delaux. P.-M., Heitmann, C., Jayaraman, D., Rajasekar, S., … & Bennett, A.B. (2018). Nitrogen fixation in a landrace of maize is supported by a mucilage-associated diazotrophic microbiota. PLoS Biology, 16(8), e2006352. https://doi.org/10.1371/journal.pbio.2006352
van Niftrik, L., & Jetten, M. S. M. (2012). Anaerobic ammonium-oxidizing bacteria: Unique microorganisms with exceptional properties. Microbiology and Molecular Biology Reviews, 76(3), 585–596. https://doi.org/10.1128/mmbr.05025-11
Wang, Z., Saski, C., Williamson, C., Campbell, B., & Ye, R. (2024). Crop cover and manure compost: Their varied effects on nitrogen availability and nitrogen cycling functional gene abundances in sandy soils for organic farming. Applied Soil Ecology, 200, 105446. https://doi.org/10.1016/j.apsoil.2024.105446
Wendeborn, S. (2020). The chemistry, biology, and modulation of ammonium nitrification in soil. Angewandte Chemie International Edition, 59(6), 2182–2202. https://doi.org/10.1002/anie.201903014
Wheatcroft, R., & Watson, R. J. (1988). Distribution of insertion sequence ISRm1 in Rhizobium meliloti and other gram-negative bacteria. Microbiology, 134(1), 113–121. https://doi.org/10.1099/00221287-134-1-113
World Resources Institute. (2022). Climate watch historical GHG emissions. https://www.Climatewatchdata.Org/Ghg-Emissions
Yang, S. S., Lai, C. M., Chang, H. L., Chang, E. H., & Wei, C. B. (2009). Estimation of methane and nitrous oxide emissions from paddy fields in Taiwan. Renewable energy, 34(8), 1916–1922.
Ye, J., Perez, P. G., Zhang, R., Nielsen, S., Huang, D., & Thomas, T. (2018). Effects of different C/N ratios on bacterial compositions and processes in an organically managed soil. Biology and Fertility of Soils, 54(1), 137–147. https://doi.org/10.1007/s00374-017-1246-5
Ye, R., Parajuli, B., Szogi, A. A., Sigua, G. C., & Ducey, T. F. (2021). Soil health assessment after 40 years of conservation and conventional tillage management in Southeastern Coastal Plain soils. Soil Science Society of America Journal, 85(4), 1214–1225. https://doi.org/10.1002/saj2.20246
Yin, S. X., Chen, D., Chen, L. M., & Edis, R. (2002). Dissimilatory nitrate reduction to ammonium and responsible microorganisms in two Chinese and Australian paddy soils. Soil Biology and Biochemistry, 34(8), 1131–1137. https://doi.org/10.1016/S0038-0717(02)00049-4
Yoneyama, T., Terakado-Tonooka, J., Bao, Z., & Minamisawa, K. (2019). Molecular analyses of the distribution and function of diazotrophic rhizobia and methanotrophs in the tissues and rhizosphere of non-leguminous plants. Plants, 8(10), 408.
Zhang, X., Ward, B. B., & Sigman, D. M. (2020). Global nitrogen cycle: Critical enzymes, organisms, and processes for nitrogen budgets and dynamics. Chemical Reviews, 120(12), 5308–5351. https://doi.org/10.1021/ACS.CHEMREV.9B00613
Self-study CEU Quiz
Earn 1.5 CEUs in Nutrient Management by taking the quiz for the article at https://web.sciencesocieties.org/Learning-Center/Courses. For your convenience, the quiz is printed below. The CEU can be purchased individually, or you can access as part of your Online Classroom Subscription.
1. Which forms of nitrogen have a high potential for leaching underground?
a. Ammonium
b. Nitrate.
c. Organic nitrogen.
d. Nitrous oxide.
2. The breakdown of organic nitrogen to ammonium is called
a. nitrogen mineralization.
b. denitrification.
c. DNRA.
d. anammox.
3. Nitrogen fixation is common in
a. Grass.
b. Cereals.
c. Brassica.
d. Legumes.
4. Low nitrate conditions with a high CN ratio favor DNRA over denitrification.
a. True.
b. False.
5. A soil organic matter CN ratio of 15 means
a. 15 units of carbon released from decomposition releases 1 unit of nitrogen.
b. 15 units of nitrogen released from decomposition releases 1 unit of carbon.
c. 15 grams of soil organic matter decomposition releases 1 gram of carbon.
d. 15 grams of soil organic matter decomposition releases 1 gram of nitrogen.
6. The stepwise denitrification is
a. nitrate to nitrite to nitrous oxide to nitric oxide to nitrogen gas.
b. nitrogen gas to nitrous oxide to nitrite to nitric oxide to nitrate.
c. nitrate to nitrite to nitric oxide to nitrous oxide to nitrogen gas.
d. nitrogen gas to nitric oxide to nitrate to nitrous oxide to nitrite.
7. BNI stands for
a. biological nitrogen increment.
b. biological nitrification inhibitor.
c. biological nitrogen inducement.
d. biological nitrogen involvement.
8. Nitrogen mineralization is tightly coupled with atmospheric nitrogen gas.
a. True.
b. False.
9. Ammonia-oxidizing archaea are involved in
a. nitrogen fixation.
b. nitrification.
c. denitrification.
d. DNRA.
10. Nitrapyrin and DMPP are common
a. nitrification enhancers.
b. denitrification enhancers.
c. nitrification inhibitors.
d. nitrogen fixation enhancers.
11. Anaerobic condition favors
a. denitrification.
b. nitrification.
c. leaching.
d. runoff.
12. Aminization is a process of converting
a. nitrogen gas to nitrous oxide.
b. nitrous oxide to nitrogen gas.
c. proteins to amino acids.
d. amino acids to protein.
13. Higher nitrogen mineralization occurs from the soil organic matter with a CN ratio of
a. 15.
b. 30.
c. 35.
d. 50.
14. Wastewater treatment plants utilize the __________ process to remove ammonium from water.
a. DNRA
b. ammonia-oxidation
c. nitrification
d. anammox
15. DNRA is
a. a reduction process.
b. an oxidation process.
c. an assimilation process.
d. a decomposition process.
Text © . The authors. CC BY-NC-ND 4.0. Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.