4R Nutrient Management for Magnesium

The principles of 4R nutrient stewardship apply to every plant nutrient. Magnesium (Mg), traditionally considered a secondary macronutrient, is often overlooked. This article reviews the options available for managing magnesium and the benefits you might expect. Earn 0.5 CEUs in Nutrient Management by reading this article and taking the quiz at https://web.sciencesocieties.org/Learning-Center/Courses.
The principles of 4R nutrient stewardship apply to every plant nutrient. Magnesium (Mg), traditionally considered a secondary macronutrient, is often overlooked. This article reviews the options available for managing magnesium and the benefits you might expect.
Magnesium is considered a secondary macronutrient. However, the term "secondary" does not diminish its importance. All macronutrients, including nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), and sulfur (S), are necessary in large quantities for crops, hence their classification as "macro-nutrients." Magnesium, along with Ca and S, is categorized as "secondary" because the soil naturally supplies much of the nutrient needed by the crop, requiring smaller amounts of fertilizer to optimize yields.
Soil Magnesium
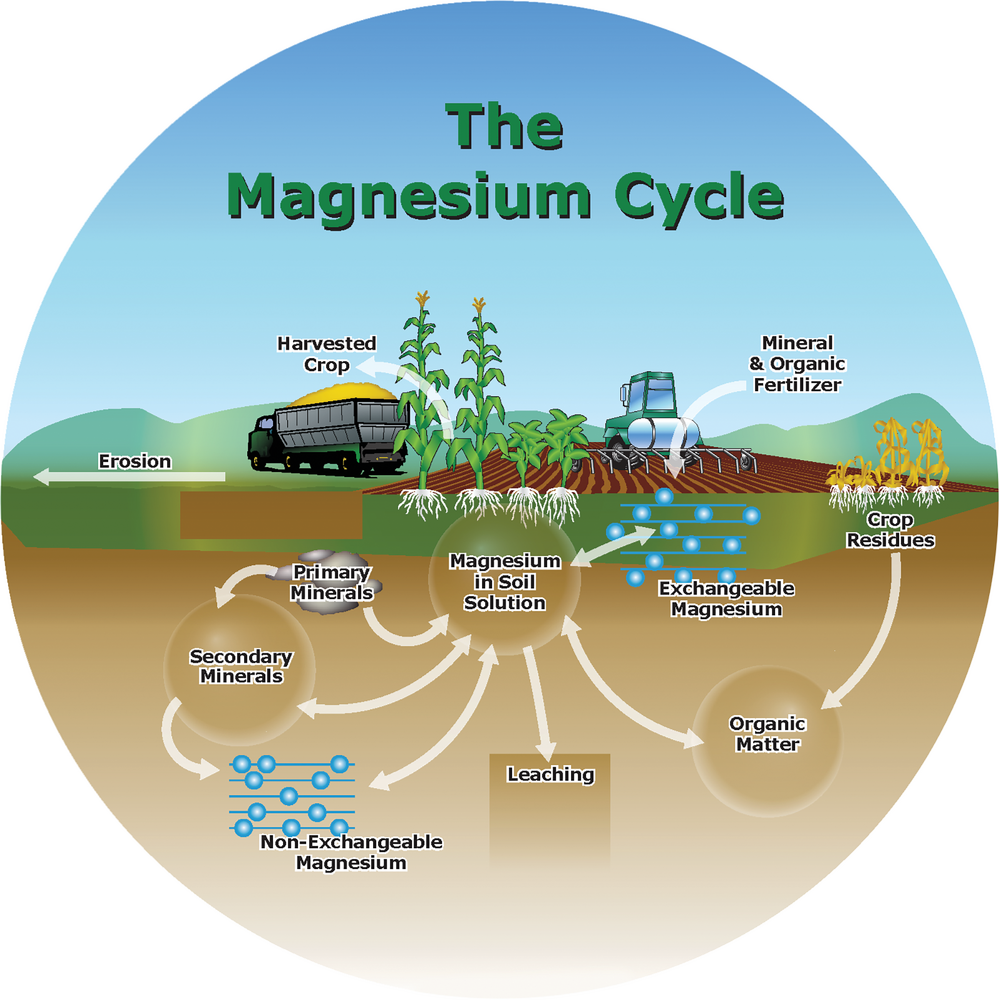
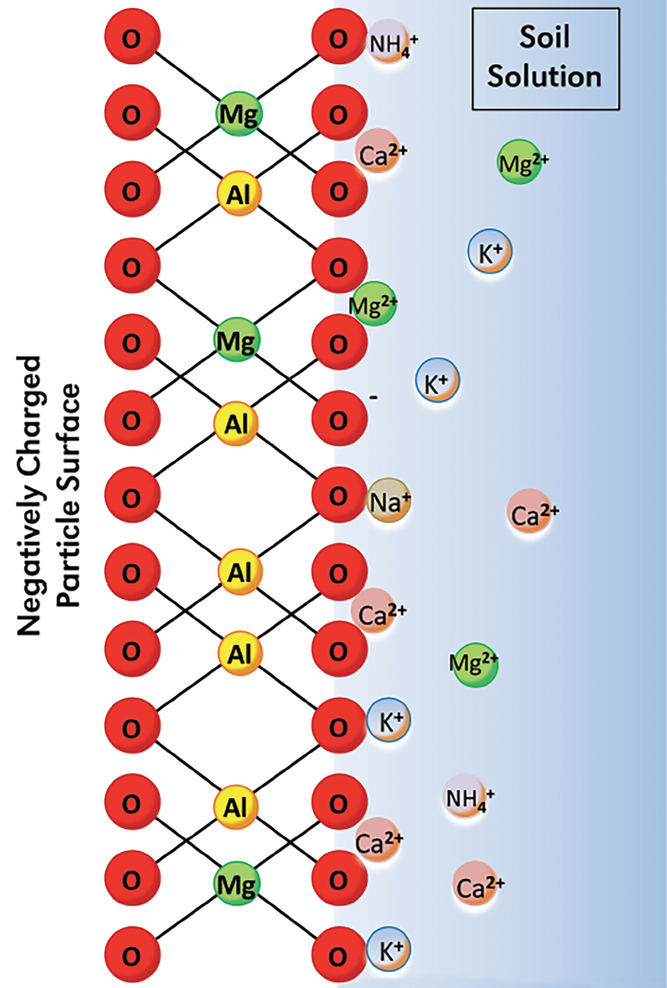
Magnesium behaves a lot like Ca in the soil. It is always present as the divalent Mg2+ cation in the soil solution and is adsorbed on cation exchange sites. It is primarily supplied to plants via mass flow, meaning that Mg2+ moves to plant roots with soil water. Magnesium is part of many soil minerals, including biotite, dolomite, hornblende, olivine, and serpentine, and the clay minerals chlorite, illite, montmorillonite, and vermiculite. It becomes available for plants as these minerals weather and break down. The plant availability of Mg is largely based on the soil’s geologic parent material. Some soils naturally have an adequate amount of Mg to supply plant growth. However, plant-available Mg tends to be low in sandy acidic soils in rainy climates.
Many other nutrients, particularly those forming cations, antagonize crop uptake of Mg. In acid soils, Al3+ and Mn2+ can reduce Mg uptake. In other soils, Ca2+, K+, and Na+ compete against Mg2+ for uptake. Where higher rates of NH4+-N have been applied, either with fertilizers or manures, Mg uptake in plants is reduced. These antagonisms are most likely to occur where soil Mg is marginal.
Plant Magnesium
In the plant, Mg behaves much differently than Ca. Typically, plant dry matter contains 0.1 to 0.8% Mg. One atom of Mg occupies the center of every chlorophyll molecule, accounting for about one- fifth of the plant’s total Mg. Magnesium stabilizes the ribosomes where proteins are synthesized, and it activates the enzymes involved in carbohydrate metabolism. It is an important element to key energy processes for plants, such as photosynthesis, the Krebs cycle, and respiration. Owing to its mobility, plants translocate Mg to where it is needed most, so deficiency symptoms show first on lower leaves. In Mg-deficient plants, generally the leaves turn yellow between the veins, a phenomenon known as interveinal chlorosis. The reasons for the yellowing relate most closely to effects on carbohydrate metabolism and transport, rather than chlorophyll shortage (deficient plants tend to have a great proportion of their Mg in the chlorophyll).
Rates
A primary decision for Mg fertilizer is whether there is a need to apply. Many North American soils are more than adequate in available Mg, as indicated in the 2020 soil test survey (TFI, 2020). Magnesium analysis is often included in a routine soil test and results show that 80% of soils contain more than 100 ppm exchangeable Mg, a concentration that exceeds the needs of most crops. Exceptions include the states of Florida, Georgia, and South Carolina, where more than 40% of soils contain less than 50 ppm exchangeable Mg, and Alabama, Alaska, and Rhode Island, with more than 25% of soils testing this low.
A decision on whether to apply Mg can be supported by scouting crops for deficiency symptoms, plant tissue testing, and/or soil testing. Target plant tissue concentrations cereal crops are 0.2–0.4 % Mg, while oilseeds like canola require 0.4 to 0.5% Mg and alfalfa requires 0.3 to 0.8% Mg. In most soils, Mg2+ occupies 4 to 20% of the cation exchange sites. In soils with serpentine parent material, excessive Mg can cause nutrient imbalances, but toxicities are rare.
Harvest of typical yields of grain and oilseed crops removes 10 to 20 lb Mg/ac. Where soils are low in Mg owing to acidity, the rate for correcting the soil pH using dolomitic limestone would normally provide enough sufficient Mg to replenish many years of crop removal.
Sources
Where low soil pH is the driver of Mg deficiency, some of the most common sources of Mg are dolomitic limestone or hydrated dolomite (Table 1). Byproducts containing Mg oxide may also supply Mg while correcting pH in acidic soils. Where soil pH is neutral or higher, these sources will not correct a Mg deficiency since they dissolve very slowly. In those cases, soluble sources like potassium-magnesium sulfate (made from the mineral langbeinite), Epsom salts, magnesium nitrate, or others shown in Table 1 may be best suited.
Table 1. Commercial sources of magnesium fertilizer.
Fertilizer name | Chemical formula | Typical Mg |
|---|---|---|
Dolomitic limestone | MgCO3*CaCO3 | 6–12 |
Hydrated dolomite | MgO*CaO / MgO*Ca(OH)2 | 18–20 |
Magnesium oxide | MgO | 56 |
Kainite | MgSO4*KCl*3H2O | 9 |
Kieserite | MgSO4*H2O | 17 |
Langbeinite | K2SO4*2MgSO4 | 11 |
Magnesium chloride | MgCl2 | 25 |
Magnesium nitrate | Mg(NO3)2*6H2O | 9 |
Magnesium sulfate | MgSO4*7H2O | 9 |
Schoenite | K2SO4*MgSO4*6H2O | 6 |
Struvite | MgNH4PO4*6H2O | 10 |
Timing and Placement
Finely ground dolomitic limestone should usually be broadcast and mixed well into the topsoil before a crop is planted. Other forms can be placed in bands near the seed row; the maximum rate that may be applied varies with the salt index of the material. In citrus trees, Mg deficiency is often corrected using foliar sprays of magnesium sulfate or magnesium nitrate.
Foliar applications are sometimes recommended for forage crops where Mg concentrations in plant tissues are too low for animal nutrition. Foliar applications are generally repeated since Mg is taken up in fairly large quantities.
Cation Ratios
Some have suggested that Mg or Ca should be applied to achieve an “ideal ratio” of Ca:Mg in the soil. This concept of an optimal basic cation saturation ratio (BCSR) emerged from work conducted in New Jersey by Bear et al. (1945). Many researchers have attempted to validate the optimal BCSR concept but have failed to find a positive crop response to changes to the ratio (Favaretto et al., 2008; Stevens et al., 2005; Reid, 1996, McLean et al., 1983). Two reviews of the body of research indicate that crop yields are not impacted by changes to basic cation ratios, and the data does not support the use of BCSR (Kopittke and Menzie, 2007; Changanti and Culman, 2017). Crops adapt well to a wide range of Ca:Mg ratios, and the ratio of Ca:Mg should not be used to drive fertilizer applications. Rather, growers should ensure their soils have an adequate supply of both Ca and Mg for specific crop needs, and then add lime to optimize yields.
The primary benefit of Mg application where soils are deficient is increased yield. When there are clear symptoms of deficiency, confirmed by plant or soil analysis, crop yields generally respond well to application of the appropriate form of Mg.
Benefits to crop quality may also be important. In their extensive review of the significance of Mg for crop quality, Gerendás & Führs (2013) noted that Mg can be considered “the forgotten element” in view of the limited number of studies on Mg compared to those for other nutrients. Their review covered a wide range of agricultural and horticultural crops, including cereals, oil crops, pulses, fruits, and vegetables. A notable example was potatoes, which had several quality attributes that improved when Mg was applied beyond the rate producing maximum yield. However, the concentration of toxic glycoalkaloids also increased when the Mg supply exceeded the rate needed for maximum yield.
Benefits
The primary benefit of Mg application where soils are deficient is increased yield. When there are clear symptoms of deficiency, confirmed by plant or soil analysis, crop yields generally respond well to application of the appropriate form of Mg.
Benefits to crop quality may also be important. In their extensive review of the significance of Mg for crop quality, Gerendás & Führs (2013) noted that Mg can be considered “the forgotten element” in view of the limited number of studies on Mg compared to those for other nutrients. Their review covered a wide range of agricultural and horticultural crops, including cereals, oil crops, pulses, fruits, and vegetables. A notable example was potatoes, which had several quality attributes that improved when Mg was applied beyond the rate producing maximum yield. However, the concentration of toxic glycoalkaloids also increased when the Mg supply exceeded the rate needed for maximum yield.
For horticultural crops in general, Gerendás & Führs (2013) noted that:
(1) the Mg/Ca ratio mainly determines functional properties, such as product firmness, texture, and storability, and
(2) the Mg/K ratio appears to influence taste and sensory properties through cellular cation/anion balances, juice acidity, and total soluble solids.

Magnesium itself in crop products serves as a nutrient for both animals and humans. It is an essential nutrient for several metabolic pathways, nerve control, and bone formation. The concentration of Mg in human diets is noted to be decreasing over time. This is true for other mineral nutrients as well, owing to shifts in diets toward more processed foods and overall declining trends in mineral nutrients as yields have increased. Decreases in human mineral nutrient intake, including that of Mg, is thought to be associated with negative health outcomes (Rosanoff, 2013; White & Broadley, 2009).
Grass Tetany and Forage Production
Low Mg in forages—particularly driven by imbalances with Ca2+, K+, and NH4+—has long been associated with grass tetany in grazing animals. Grass tetany, or hypomagnesemia, is an abnormally low concentration of Mg in the blood, which can be fatal for grazing animals. Grass tetany is a specific concern for transitioning or lactating cattle. Typical symptoms include stiff legs, stiff neck, falling, or tremors. Grass tetany is often associated with lush early spring growth, but can occur when feeding ensiled, baled, and grazed forages any time of the year.
Plant uptake of Mg is directly influenced by the interaction of exchangeable Mg, Ca, and K in soils. Greater soil K concentrations are known to depress Mg uptake in forages. Furthermore, forages rich in K or soluble nitrogen (NH4+) can disrupt Mg absorption in rumen in cattle. Ultimately, low Mg concentrations in forages, and competition for absorption sites in the rumen can all contribute to grass tetany. Grass tetany occurs most often when forages are grown on acidic soils, or soils low in Mg and high in K, Ca, and/or soluble nitrogen. Best practices to avoid grass tetany are to test forages for their concentrations of Ca, K, and Mg; to supplement with dietary Mg; and to test soils used for forage production or grazing. Many grasses are “luxury” consumers of K, so K fertilizer should be applied to recommend recommended rates and not in excess. Cool temperatures also favor plant uptake of K over Mg. When liming, use a dolomitic lime, which will supply both Ca and Mg, rather than calcitic lime, which does not contain Mg.
In addition to preventing grass tetany, increasing prenatal dietary Mg concentrations were shown to have a large effect decreasing the incidence of milk fever (Lean et al., 2006).
Best Practices for Magnesium Management
Magnesium is an important macronutrient for crop growth and should not be overlooked in 4R nutrient management. While it is a secondary macronutrient, Mg plays critical roles in various plant metabolic processes, including photosynthesis. An adequate supply of Mg is necessary to optimize crop yields. Understanding the dynamics of soil Mg concentrations, nutrient interactions, and appropriate application methods are essential for effective 4R management. Special attention should be given to Mg in forage production to prevent grass tetany and ensure balanced nutrition in forages.
References
Bear, F.E., Prince, A.L., & Malcolm, J.L. (1945). Potassium needs of New Jersey soils. New Jersey Agricultural Experiment Station.
Bear, F.E., & Toth, S.J. (1948). Influence of calcium on availability of other cations. Soil Science, 65, 69–74. https://doi.org/10.1097/00010694-194801000-00007
Chaganti, V.N., & Culman, S.W. (2017). Historical Perspective of Soil Balancing Theory and Identifying Knowledge Gaps: A Review. Crop, Forage & Turfgrass Management, 3, 1–7. https://doi.org/10.2134/cftm2016.10.0072
Favaretto, N., Norton, L.D., Brouder, S.M., & Joern, B.C. (2008). Gypsum amendment and exchangeable calcium and magnesium effects on plant nutrition under conditions of intensive nutrient extraction. Soil Science, 173(2), 108–118. https://doi.org/10.1097/SS.0b013e31815edf72
Gerendás, J., & Führs, H. (2013). The significance of magnesium for crop quality. Plant Soil, 368(1), 101–128. https://doi.org/10.1007/s11104-012-1555-2
Kopittke, P.M., & Menzies, N.W. (2007). A review of the use of the basic cation saturation ratio and the “ideal” soil. Soil Science Society of America Journal, 71, 259–265. https://doi.org/10.2136/sssaj2006.0186
Lean, I.J., DeGaris, P.J., McNeil, D.M., & Block, E. (2006). Hypocalcemia in dairy cows: meta analysis and dietary cation anion difference theory revisited. Journal of Dairy Science, 89(2), 669–684. https://doi.org/10.3168/jds.S0022-0302(06)72130-0.
McLean, E.O., Hartwig, R.C., Eckert, D.J., & Triplett, G.B. (1983). Basic cation saturation ratios as a basis for fertilizing and liming agronomic crops: II. Field studies. Agronomy Journal, 75, 635–639. Mikkelsen, R. (2010). Soil and fertilizer magnesium. Better Crops, 94(2), 26– 8. http://www.ipni.net/publication/bettercrops.nsf/issue/BC-2010-2
Reid, W.S., (1996). Influence of lime and calcium:magnesium ratio in alfalfa and birdsfoot trefoil yields. Communications in Soil Science and Plant Analysis, 27(5–8), 1885–1900. https://doi.org/10.1080/00103629609369676
Rosanoff, A. (2013). Changing crop magnesium concentrations: impact on human health. Plant and Soil 368(1), 139–153. https://doi.org/10.1007/s11104-012-1471-5
Stevens, G., Gladbach, T., Motavalli, P., & Dunn, D. (2005). Soil calcium: magnesium ratios and lime recommendations for cotton. The Journal of Cotton Science, 9, 65–71.
White, P.J., & Broadley, M. R. (2009). Biofortification of crops with seven mineral elements often lacking in human diets--iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytologist 182(1), 49–84. https://doi.org/10.1111/j.1469-8137.2008.02738.x
Self-Study CEU Quiz
Earn 1 CEU in Nutrient Management by taking the quiz for the article at https://web.sciencesocieties.org/Learning-Center/Courses. For your convenience, the quiz is printed below. The CEU can be purchased individually, or you can access as part of your Online Classroom Subscription.
- The Soil Test Survey indicated that ___% of soils contain more than ____ ppm exchangeable Mg, which exceeds crop need for most crops.
- 75 and 100
- 80 and 100
- 75 and 90
- 80 and 90
- What is one of the primary symptoms of magnesium deficiency in plants?
- Wilting leaves
- Necrotic leaves
- Interveinal chlorosis
- Stunted root growth
- What is the primary method by which magnesium is supplied to plants in the soil?
- Root interception
- Atmospheric deposition
- Mass flow with soil water
- Photosynthesis
- Which of the following is least useful for guiding Mg fertilizer applications:
- Soil test Mg levels
- Plant tissue testing
- Soil basic cation saturation ratio (BCSR)
- Forage K, Ca, and Mg concentrations
- Grass tetany, or hypomagnesemia, in grazing animals is most likely to occur under which conditions?
- High Mg levels in soil
- Low K levels in forages
- Acidic soils with low Mg levels
- Excessive Mg supplementation in diets
EXTRA Questions
- What is the recommended range of plant magnesium concentrations in cereal crops?
- 0.1 - 0.3%
- 0.2 - 0.4%
- 0.5 - 0.7%
- 0.8 - 1.0%
- In addition to reducing grass tetany, what other effect does increasing dietary magnesium concentrations have?
- Decreases the incidence of milk fever
- Decreases milk production
- Increases calcium absorption
- Decreases potassium absorption
- Which of the following soil minerals does not contain magnesium?
- Biotite
- Olivine
- Quartz
- Dolomite
- Order the following sources from the lowest Mg concentration to highest Mg concentration (lowest on left to highest on right):
- Magnesium sulfate < Struvite < Hydrated dolomite < Magnesium oxide
- Struvite < Magnesium sulfate < Magnesium oxide < Hydrated dolomite
- Magnesium sulfate < Struvite < Magnesium oxide < Hydrated dolomite
- Struvite < Magnesium sulfate < Dolomite < Magnesium oxide
Text © . The authors. CC BY-NC-ND 4.0. Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.










