Palmer Amaranth in the Pacific Northwest


Palmer amaranth is one of the most troublesome pigweeds in crop production systems in the United States. It only recently started to appear in the Pacific Northwest (PNW). A coordinated extension and outreach effort among land grant universities (University of Idaho, Oregon State University, and Washington State University), Amalgamated Sugar, other commodity commissions, and industry was launched to track Palmer amaranth in the PNW. In 2023, tissue samples were collected from pigweeds suspected to be Palmer amaranth with tests providing confirmation. Palmer amaranth was detected in several crops as well as right-of-way and private property. Most of the Palmer amaranth populations were confirmed to be resistant to glyphosate. Additional surveys and resistance screening efforts are underway to map the distribution of Palmer amaranth and assess the level of herbicide resistance across the region. Earn 0.5 CEUs in Integrated Pest Management by reading this article and taking the quiz at https://web.sciencesocieties.org/Learning-Center/Courses.
Palmer amaranth is arguably the most troublesome pigweed in crop production systems in the United States. Although native to the southwest United States, this pigweed rose from obscurity to stardom within the past three decades. There are various hypotheses on what factors contribute to the continued spread of Palmer amaranth in the U.S. These include human-aided spread, climate change, shifts in habitat preference, and rapid evolution. There’s no general agreement on which factors are the primary drivers, but it is clear that these factors are acting in tandem to favor the spread of Palmer into agricultural production systems. Aside from the continued spread of this pigweed, Palmer amaranth has evolved resistance to multiple herbicide sites of action across several states where this weed has been established. Thus, Palmer amaranth introduced into new states or habitats is likely to bring with it resistance to commonly used herbicides.
A Quick Guide to Palmer Amaranth Identification
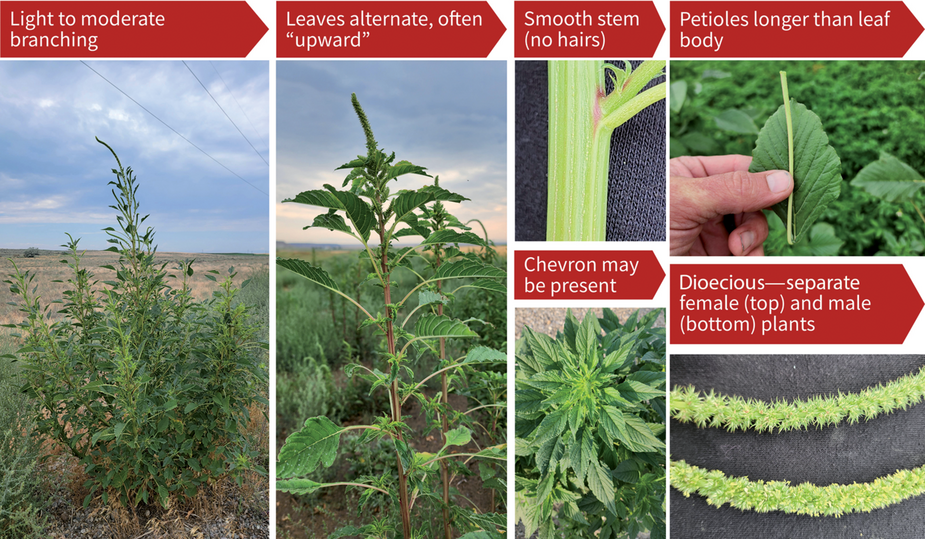
Pigweeds can be incredibly similar during their seedling and vegetative growth stages. However, there are a few features, such as leaf shape, size, length, and the presence or absence of watermarks (chevron), that may help differentiate young Palmer amaranth from other pigweeds. While some of the features shown below are unique to Palmer amaranth and the other pigweeds, it must be noted that the appearance of any given weed tends to vary based on the growing conditions and location. Therefore, relying on multiple features or characteristics is advisable for correct identification.
Palmer amaranth has an oval, diamond, or lance-shaped leaf (Figure 1). The length of Palmer amaranth petioles (the stemlike structure that connects the leaf blade to the main stem) can be more than three inches long, which is longer than the leaf blade. This is not the case with other pigweeds. The leaves of Palmer amaranth tend to have a V-shaped watermark, often referred to as a chevron, although not always (Figure 2). Palmer amaranth has smooth stems that often lack hair.
In Palmer amaranth, male and female flowers develop on different plants (dioecious). The male flowers are soft to the touch, while the female flowers are very spiny. Palmer amaranth flowers are produced in groups of flowers at the ends of upper branches, and the flowers attach directly to the stem.
Factors Favoring Spread and Establishment Across Different States
Palmer amaranth is native to the desert, and for years it has been thought that this weed cannot spread to states with colder climates and shorter frost-free growing days. The eastward and northward movement of this species has proven that Palmer amaranth has evolved to tolerate colder climates over the years. Studies have shown that Palmer amaranth can germinate within a wide range of soil temperatures (Figure 3).
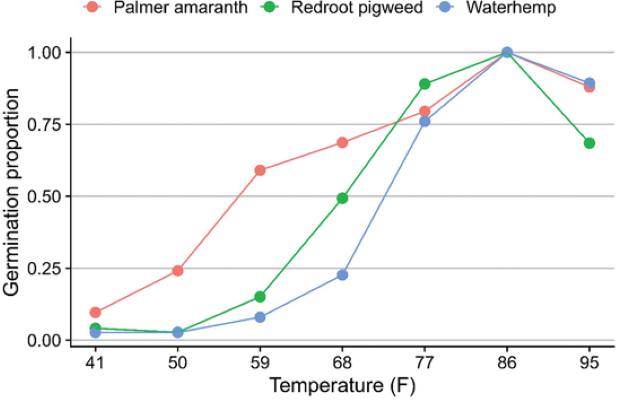
This wide temperature window for Palmer amaranth emergence favors multiple flushes of the weed within a single growing season. This makes it difficult for growers to effectively control Palmer amaranth, especially those who rely solely on postemergence herbicides. Even where effective postemergence herbicides are available, multiple applications are often required to provide season-long weed control. In crops such as sugar beet where the only effective postemergence herbicide is glyphosate, farmers are forced to apply glyphosate multiple times within a single growing season. This has resulted in intense selection pressure for herbicide resistance.
Herbicide Resistance
The ability to rapidly evolve resistance to multiple herbicide sites of action is by far the most important factor contributing to the impact of this weed in cropping systems. One consequence is increased time, effort, and cost of control. According to data submitted to The International Herbicide-Resistant Weed Database (http://www.weedscience.org) (Heap, 2024), more than 60 unique cases of herbicide resistant Palmer amaranth populations have been reported in several states across the U.S. (Figure 4). This involves at least nine herbicide sites of action (Table 1). What makes this concerning is that this encompasses nearly all the major herbicide groups used in commonly grown crops in the Pacific Northwest (PNW).
Table 1. Herbicide resistance in Palmer amaranth in the United States.
Site of Action (aHRAC#) | Example of active ingredient (Trade name) |
|---|---|
Acetolactate synthase (ALS) inhibitors (2) | Rimsulfuron (Matrix); imazethapyr (Pursuit) |
Microtubule assembly inhibitors (3) | Ttrifluralin (Treflan) |
Auxin mimics (4) | 2, 4-D (2,4-D Ester); dicamba (Clarity) |
Photosystem II inhibitors (5) | Atrazine (Aatrex) |
Enolpyruvyl shikimate phosphate synthase (EPSPS) inhibitors (9) | Glyphosate (Roundup PowerMax) |
Glutamine synthetase inhibitor (10) | Glufosinate (Liberty) |
Protoporphyrinogen oxidase (PPO) inhibitors (14) | Fomesafen (Reflex) |
Very long-chain fatty acid synthesis inhibitors (15) | S-metolachlor (Dual Magnum) |
Hydroxyphenyl pyruvate dioxygenase (HPPD) inhibitors (27) | Mesotrione (Callisto) |
aHRAC, Herbicide Resistance Action Committee.

How was Palmer Amaranth Introduced in the Pacific Northwest?
Palmer amaranth was introduced into the PNW through various sources. These included:
Birdfeed. At least in one instance in southwest Idaho, birdfeed contaminated with Palmer amaranth seeds was identified as the source of introduction. This infestation was very small, but it was unclear how far the birds would spread the seeds.
Livestock feed. There’s a significant presence of dairy farms in the PNW, especially in southern Idaho. One of the primary feed supplements for the dairy industry is cottonseed meal. Because cotton is primarily grown in states where Palmer amaranth is widespread, these feed supplements often come contaminated with Palmer amaranth seeds. In fact, there have been widespread reports of volunteer cotton growing in Idaho and Oregon, which supports the suspicion that this supplement is one of the primary sources of introduction (Figure 5). Also, multiple Palmer amaranth populations were found near dairies in southern Idaho.
Manure. Palmer amaranth has a very hard seed coat which means that even if fed to livestock, a significant proportion could still be viable after passing through the digestive tract. Many farmers unknowingly applied manure contaminated with viable Palmer amaranth seeds, which was one of the primary sources of introduction to farms in the PNW.
Farm equipment. Palmer amaranth seeds are very small and hard to clean completely from farm equipment. We found large populations of Palmer amaranth on very secluded farms which we suspected came through used farm equipment or trucks used in hauling produce.
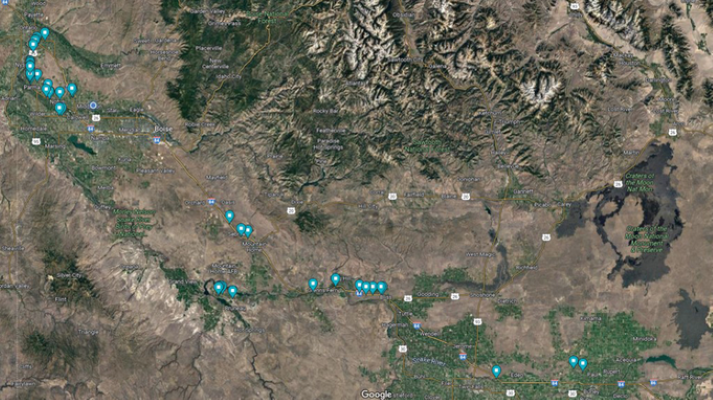
Rights-of-way. A significant number of Palmer amaranth detections in the PNW are by roadsides (Figure 6), suggesting these were introduced through contaminated vehicles traveling across the region from other states.
Distribution of Palmer Amaranth in the Pacific Northwest
Palmer amaranth has been detected in multiple counties across the PNW region (Figure 7). In Idaho, Palmer amaranth was detected in Canyon, Cassia, Elmore, Gooding, Jerome, Minidoka, and Owyhee counties. Palmer amaranth was also detected in Malheur and Marion counties in Oregon and Spokane and Walla Walla counties in Washington. The detections in Idaho and Malheur County in Oregon were large infestations that were already established. Only a few plants were detected in Washington, and these were controlled to prevent seed production. It was apparent from most of the surveys in 2023 that Palmer amaranth may be more widely distributed in the PNW than initially thought.

Situations Where Palmer Amaranth was Found in the Pacific Northwest
We found Palmer amaranth in several crops (corn, dry bean, hay, potato, small grains, sugarbeet), as well as right-of-way and private property (Figure 8). The crops most impacted were potato and sugarbeet. However, because of the diverse crop rotations in the PNW, Palmer amaranth will have a broader impact on several crops grown in the region.
Herbicide Resistance in Palmer Amaranth Found in the Pacific Northwest
We already suspected that Palmer amaranth introduced into the PNW may come from states where the weed is already resistant to a few herbicides. In the first round of greenhouse herbicide resistance bioassay, glyphosate resistance was confirmed. Some of the samples survived 44 fl oz/ac of Roundup PowerMax (Figure 9). This was not surprising as some fields surveyed showed Palmer amaranth that survived multiple applications of glyphosate (Figure 10). None of the seed collections were resistant to dicamba (XtendiMax) or glufosinate (Liberty 280 SL) (Figure 9). It is suspected that the majority of the Palmer amaranth introduced into the PNW will also be resistant to acetolactate synthase (ALS) inhibitors (group 2) herbicides since resistance to this group of herbicides is very widespread. Resistance screening of both seed and tissue samples will continue to assess whether the Palmer amaranth introduced into the PNW are resistant to any other herbicides.
Best Management Practices
Once Palmer amaranth has been successfully identified, every effort must be made to remove all plants and not allow them to produce seed even if the population is not herbicide-resistant. Integrated weed management strategies should be adopted to prevent Palmer amaranth from becoming established in new areas and preventing seed production. Several management practices are available (for example mechanical control, crop diversity and competition, herbicides, et cetera), but these would vary among crops as well as the level of herbicide resistance in the population. Avoid relying solely on herbicides for management as Palmer amaranth can rapidly evolve resistance to herbicides.
Concluding Thoughts
As with any invasive species, it is easier and more cost-effective to manage small populations before they become established in the area. Survey efforts need to be intensified to get a good picture of the distribution and herbicide resistance in the populations to provide better recommendations for management in crop and non- crop areas. In areas where Palmer amaranth has already established, it is important to effectively manage this weed in all phases of the crop rotation and adopt a zero-tolerance for escapes.
References
Adjesiwor, A.T., Prather, T., Felix, J., & Morishita, D. (2021). Pigweeds: current and emerging weed threats in the Pacific Northwest. Pacific Northwest Extension.
Heap, I. (2024). The international herbicide-resistant weed database. https://www.weedscience.org
Legleiter, T., & Johnson, B. (2013). Palmer amaranth biology, identification, and management. Purdue Extension. https://www.extension.purdue.edu/extmedia/WS/WS-51-W.pdf
Mohseni-Moghadam, M., C. Kent, & Ashigh, J. (2013). Palmer amaranth biology and management. New Mexico State University Cooperative Extension Service. https://aces.nmsu.edu/pubs/_a/A617.pdf
Pratt, D. B., M. D. K. Owen, L. G. Clark, & Gardner, A. (1999). Identification of the weedy pigweeds and waterhemps of Iowa. Iowa State University Extension and Outreach. www.extension.iastate.edu/Publications/PM1786.pdf
Sosnoskie, L. M. (2018). Pigweed identification (a quick guide). Notes in the Margins: Agronomy and Weed Science Musings. https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=27466
Steckel, L. E., Sprague, C. L., Stoller, E. W., & Wax, L. M. (2004). Temperature effects on germination of nine Amaranthus species. Weed Science, 52(2), 217-221. https://doi.org/10.1614/WS-03-012R
Tekiela, D., & Sbatella, G. (2017). Amaranthus species - A current and emerging threat in Wyoming. University of Wyoming Extension. https://www.uwyo.edu/ipm/_files/docs/ag-ipm-docs/weed-ipm-docs/amaranthus-species-a-current-and-emerging-threat-in-wyoming.pdf
Ward, S., T. Webster, & Steckel, L. (2013). Palmer amaranth (Amaranthus palmeri): A review. Weed Technology, 27(1), 12–27. https://doi.org/10.1614/WT-D-12-00113.1
Self-Study CEU Quiz
Earn 1 CEU in Nutrient Management by taking the quiz for the article at https://web.sciencesocieties.org/Learning-Center/Courses. For your convenience, the quiz is printed below. The CEU can be purchased individually, or you can access as part of your Online Classroom Subscription.
- Palmer amaranth is a pigweed.
- True.
- False.
- Which of the following is NOT one of the herbicide sites of action that Palmer amaranth has developed resistance to?
- Acetolactate synthase (ALS) inhibitors (2).
- Acetyl CoA carboxylase inhibitors (1).
- Auxin mimics (4).
- Enolpyruvyl shikimate phosphate synthase (EPSPS) inhibitors (9).
- Which of the following was NOT one of the sources through which Palmer amaranth was introduced to the Pacific Northwest?
- Birdfeed.
- Restoration seed.
- Farm equipment.
- Livestock feed.
- Which of the following is NOT a feature that may help differentiate Palmer amaranth from other pigweeds?
- The presence or absence of hairs on the stem.
- Petiole is longer than the leaf blade.
- Seed color.
- Seedhead.
- The majority of Palmer amaranth detections in the Pacific Northwest were in which of these states?
- Oregon.
- Idaho.
- Washington.
- Nevada.
Text © . The authors. CC BY-NC-ND 4.0. Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.










