The Role of Sulfur in Meeting 4R Nutrient Stewardship Goals


Sulfur plays several roles in 4R plant nutrition. First, as an essential plant nutrient, it may need to be applied to optimize yields and quality of crops. Second, there may be a need to replenish the sulfur removed from the soil by crop harvests. Third, some forms of sulfur may have additional benefits through their effects on soil pH and on soil nitrogen processes. The three roles combine to support enhanced productivity with lower impacts on the environment. This article reviews basic sulfur nutrition, recent trends affecting the need for fertilizers, and the contribution of sulfur to improving productivity sustainably. Earn 1 CEU in Nutrient Management by reading this article and taking the quiz at https://web.sciencesocieties.org/
Learning-Center/Courses.
Sulfur plays several roles in 4R plant nutrition. First, as an essential plant nutrient, it may need to be applied to optimize yields and quality of crops. Second, there may be need to replenish the sulfur removed from the soil by crop harvests. Third, some forms of sulfur may have additional benefits through their effects on soil pH and on soil nitrogen processes. The three roles combine to support enhanced productivity with lower impacts on the environment. This article reviews basic sulfur nutrition, recent trends affecting the need for fertilizers, and the contribution of sulfur to improving productivity sustainably.
Essential Role
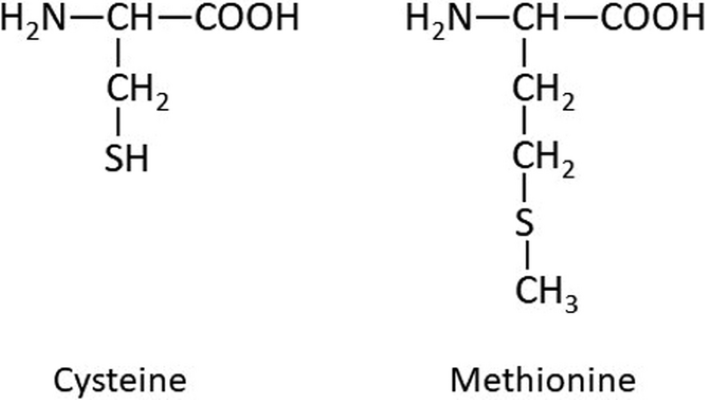
Sulfur forms part of three amino acids: cysteine, cystine, and methionine. Plants need sulfur to grow and develop. Amino acids are essential for synthesis of proteins, polypeptides, and enzymes, without which organisms cannot survive. Sulfur atoms are joined with carbon, nitrogen, oxygen, and hydrogen to form the molecular structure of amino acids (Figure 1). When plants run short of sulfur, the symptoms are like those of nitrogen deficiency. The leaves turn yellow (Figure 2). In contrast to nitrogen, symptoms start on younger leaves. Both yield and quality can be reduced with a shortage of sulfur.

Trends
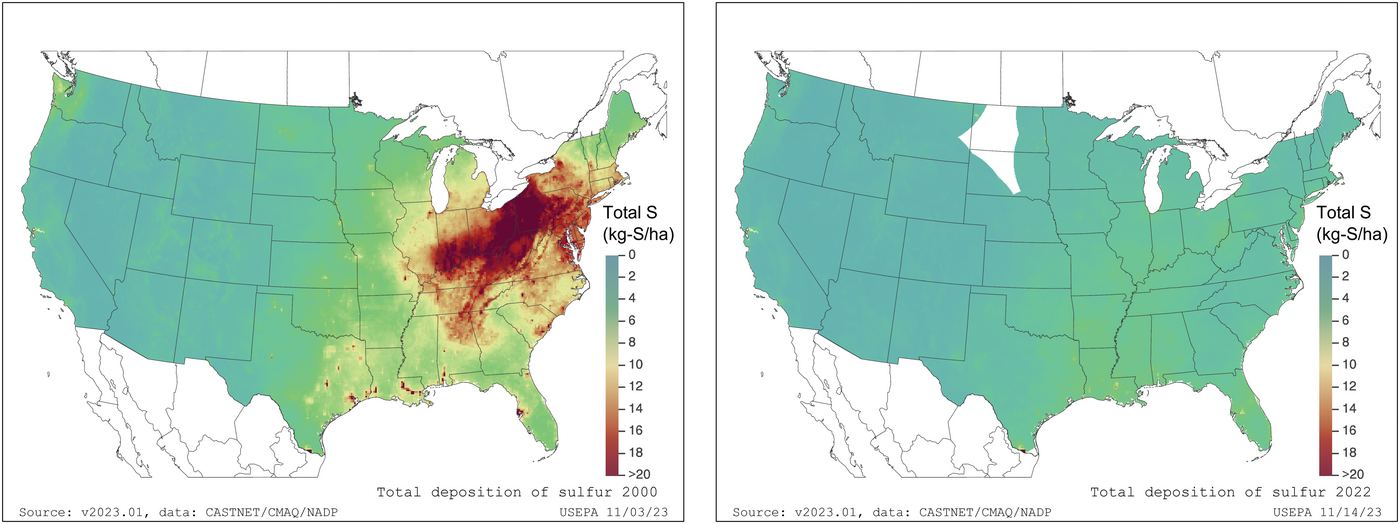
Sulfur deposition from the atmosphere was a major pollution concern four or five decades ago in the northeast part of North America. Since then, it has declined to very low levels, owing to the successful implementation of emission controls on fuels used for power generation and transport. Between 2000 and 2022, deposition dropped most dramatically from Indiana to New Jersey (Figure 3) and in the main cropland areas of southern Ontario and Quebec. Across the U.S. Midwest (Ohio to Nebraska to North Dakota), between 1985 and 2015 average rates of sulfur fertilizer application increased from 1 to 4 lb/ac of total cropland as atmospheric deposition declined from 5 to 1 lb/ac (Hinckley & Driscoll, 2022). Deposition of sulfur across the contiguous United States averaged 1.4 lb/ac for 2017 (Hinckley et al., 2000).
Recent Field Testing
As expected considering the decline in sulfur supplied from atmospheric deposition, field trials in the last ten years show an increased frequency of crop response to applied sulfur. In Indiana corn, sulfur fertilizer applied as sidedress increased yield in 26 of 55 trials from 2017 to 2022 (Camberato et al., 2023). The yield benefit in the responsive trials ranged from 3 to 34 bu/ac, with a mean of 11 bu/ac. Yield responded more frequently to sidedress sulfate than to starter applications of ammonium thiosulfate. Predicting where responses would or would not occur proved elusive.
In the province of Ontario, wheat responded more frequently to sulfur than corn or soybeans over a total of 43 sites from 2018 to 2020. Eight of 13 winter wheat trials from 2018 to 2020 showed a significant positive response – in fact, when averaged over all 13 sites, applying sulfur increased net return by $22 per acre. Generally, applying 18 lb/ac to the wheat sufficed for all three crops in the typical corn-soybean-wheat rotation (GFO, 2024). Even though responses in corn and soybeans were less frequent, these data suggest that any field receiving no other sources of sulfur—for example, those not receiving manure—should receive some sulfur fertilizer.
Recent field testing in Pennsylvania indicates potential for positive corn yield response to sulfur fertilization in the Northeastern U.S. About 20% of 27 trials from 2015 to 2017 showed a yield response to applied sulfur. There were no differences among three sources of sulfate: ammonium, potassium, or calcium. The average corn yield increase resulting from sulfur fertilizer at responsive sites was 14% (Brimmer et al., 2018).
Despite the decline in deposition from the air, however, not all areas and crops show a high frequency of response to sulfur application. An extensive set of field trials in Ohio from 2013 to 2021 showed less widespread response than expected (Fleuridor et al., 2023). Out of 96 trials, only seven responded positively: 4 out of 50 corn trials, 3 of 34 soybean trials, and none of 12 wheat trials. Thirteen on-farm replicated trials on soybean in New York from 2017 to 2019 showed little response. The researchers concluded that sulfur is not currently limiting New York soybean grain yield (Putman et al., 2021).
Across 18 site-years of field testing of sulfur applications to hard red spring wheat in Minnesota, Kaiser et al. (2019) found that 8 lb/ac of sulfur maximized grain yield and protein concentration on soils with less than 2% organic matter. Soils with higher organic matter showed no crop yield response to sulfur application. In Minnesota, sulfur is recommended for corn on sandy soils and on those with less than 4% organic matter.
The Northern Great Plains, including the Canadian Prairie Provinces, received much less sulfur deposition in the past. Over the past two or more decades, farmers have greatly increased the yields of canola and cereal crops in this region. The relatively higher need for sulfur of canola compared to cereals has been known for many decades, and a large body of research supports application of around 20 lb/ac of sulfur for optimum yields of canola (Ma et al., 2015 and literature cited therein).
Crop Uptake and Removal
When making fertilizer decisions for sulfur, it is helpful to know both the expected uptake of sulfur into the plant and the amount removed by crop harvest. The two may differ substantially. Decomposition of soil organic matter, deposition from the atmosphere, and applied organic materials and fertilizers can all contribute to the supply of sulfur for crop uptake. To replenish the soil following harvest on soils where sulfur deficiencies occur frequently, the annual supply needs to balance crop removal. Crop harvest generally removes less sulfur than nitrogen and potassium, but only a little less than phosphorus. Since sulfur concentrations can vary widely, the figures in Table 1 should be treated as general estimates only. Use locally available data wherever possible.
Table 1. Estimated total removal of sulfur (S) in the harvested portion of selected field crops compared to nitrogen (N), P2O5 and K2O, and total uptake.
Removala | Uptakeb | |||||
|---|---|---|---|---|---|---|
N | P2O5 | K2O | S | S | ||
Crop | Yield per acre | lb/ac | lb/ac | |||
| Corn | 200 bu | 134 | 70 | 50 | 10-16 | 20 |
| Soybeans | 60 bu | 195 | 44 | 72 | 11 | 20 |
| Wheat | 60 bu | 89 | 34 | 20 | 6 | 17 |
| Alfalfa | 5 ton | 255 | 60 | 245 | 27 | 30 |
| Canola | 50 bu | 80 | 40 | 20 | 12.5 | 32 |
aData from TFI (2020) and Heckman et al. (2003).
bCamberato et al. (2022) and Canola Council of Canada.
Fertilizer Sources
Many fertilizers contain sulfate and are moderately to highly water-soluble. Table 2 lists common fertilizer sources, along with their chemical formulas and sulfur contents. Soluble forms also include bisulfites, thiosulfates, and polysulfides. The water-soluble sulfates are immediately available to plants and should be used when sulfur is needed quickly. Elemental sulfur is not water-soluble and requires time in the soil for oxidation to the sulfate form. Water-dispersible (S-bentonite) and micronized forms release smaller particles that oxidize more rapidly. Although gypsum (calcium sulfate) is less water-soluble than many other sulfate fertilizers, it can be a sufficiently available, effective, and inexpensive sulfur source in some regions.
Fertilizer material | Chemical formula | Sulfur | Form |
|---|---|---|---|
| Ammonium sulfate | (NH4)2SO4 | 24 | Solid |
| Ammonium thiosulfate | (NH4)2S2O3•5H2O | 26 | Fluid |
| Ammonium polysulfide | (NH4)2Sx | 40-50 | Solid |
| Potassium sulfate | K2SO4 | 18 | Solid |
| Potassium-magnesium sulfate | K2SO4•2MgSO4 | 22 | Solid |
| Elemental sulfur | S | >85 | Solid |
| Gypsum | CaSO4•2H2O | 12-18 | Solid |
| Magnesium sulfate | MgSO4•7H2O | 14 | Solid |
| Potassium thiosulfate | K2S2O3 | 17 | Fluid |
Fertilizers are expressed on their S content, but plants take up the sulfate ion. To convert S to sulfate, multiply by 3; to convert sulfate to S, divide by 3.
Soil Testing
A survey of soil testing laboratories found that from 2005 to 2020, a greater proportion of soil samples tested low in sulfur (TFI, 2020). The declining trend in soil test sulfur is consistent with lower deposition of sulfate from the atmosphere. Only a few areas, however, show more than 20% of soil samples testing lower than 3 parts per million (ppm) calcium phosphate equivalent sulfur. These include the province of Quebec and the states Georgia, Indiana, Louisiana, Wisconsin, Illinois, Arkansas, Florida, and Mississippi. Unfortunately, soil tests for sulfur are not well correlated to probabilities of yield response, so agronomic interpretations are not very clear. The most used soil test is the calcium phosphate method. The critical level for sulfate-S by that method is often taken to be 3 ppm, or an equivalent of 6 ppm for a Mehlich-3 soil test.
Additional Benefits
Ammonium sulfate as a form of nitrogen generates more soil acidity—about 50% more per unit of nitrogen applied—than other common forms of nitrogen fertilizer like urea, anhydrous ammonia, and ammonium nitrate. This can be a concern in acidic soils but may be a benefit in soils of higher pH where the acidity generated may increase the availability of nutrients like phosphorus, manganese, and zinc, particularly when applied in a band.
Elemental sulfur also generates substantial acidity as it oxidizes. Each pound applied neutralizes about six pounds of calcium carbonate. While this is twice as much acidity, pound for pound of sulfur versus nitrogen, typical agronomic rates have minimal effect on bulk soil pH in calcareous soils with pH of 7.5 or higher. The 4R Nutrient Stewardship framework includes monitoring soil pH and applying lime where needed. Lime is more likely to be needed to counter the acidifying effects of nitrogen and sulfur fertilizers in sandier soils with pH below 7. Gypsum and other sulfate forms that contain no ammonium generate no acidity, and neither increase nor reduce soil pH. In cropland soils, the degradation that sulfur depositions created on forest soils may be mitigated through liming practices and the application of other fertilizers (Hinckley et al., 2020).
The thiosulfate forms have been shown to inhibit nitrification and nitrous oxide (N2O) emissions, and to a lesser extent slow ammonia loss by inhibiting urea hydrolysis. Cai et al. (2018) demonstrated in a laboratory incubation that rates of potassium thiosulfate supplying 45 to 180 lb/ac sulfur could reduce N2O emissions by 25%–48%. Several field studies on rice, wheat, and tomatoes in India have reported N2O emission reductions of 9%–35% in response to mixing thiosulfate with urea. These studies generally had low rates (<1 kg/ha) of N2O emission, however. Compared to other nitrification inhibitors, thiosulphates show less efficacy in reducing emissions. In the meta-analysis of Akiyama et al. (2010), thiosulfates showed 40% and 65% of the efficacy of DMPP and DCD, respectively. Nevertheless, inhibition of nitrification in many situations may additionally reduce nitrate leaching as well as losses of nitrate by denitrification.
Summary
Sulfur nutrition is important to the yield and quality of crops. Deficiencies are most likely on soils of sandier texture, where crops are irrigated, and where amounts of sulfur deposited from the air have declined relative to the 1970s and 1980s. As yields of crops increase, the amount of sulfur removed increases as well and may need replenishment. Various forms of sulfur fertilizer can be used to control soil pH for better soil health and to control nitrogen transformation processes to limit losses of nitrate and nitrous oxide, reducing the environmental footprint of crop production. Crop sulfur nutrition therefore plays several important roles in 4R nutrient stewardship.
Acknowledgment
Appreciation is expressed to Tom Jensen for review.
References
Akiyama, H., Yan, X., & Yagi, K. (2010). Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: Meta-analysis. Global Change Biology, 16(6), 1837–1846. https://doi.org/10.1111/j.1365-2486.2009.02031.x.
Brimmer, R., Beegle, D. B., White, C. M., & Spargo, J. T. (2018). Predicting sulfur needs of corn in Pennsylvania using soil testing and plant tissue analysis. 2018 ASA and CSSA Annual Meeting, Baltimore, MD. https://scisoc.confex.com/scisoc/2018am/meetingapp.cgi/Paper/112703
Cai, Z., Gao, S., Xu, M., & Hanson, B.D. (2018). Evaluation of potassium thiosulfate as a nitrification inhibitor to reduce nitrous oxide emissions. Science of The Total Environment, 618, 243–249. https://doi.org/10.1016/j.scitotenv.2017.10.274.
Camberato, J., Casteel, S., & Steinke, K. (2022). Sulfur deficiency in corn, soybean, alfalfa, and wheat. Purdue UnivERSITY Extension https://www.extension.purdue.edu/extmedia/AY/AY-379-W.pdf.
Camberato, J., Nielsen, B., Salguero, D., & Quinn, D. (2023). Corn response to sulfur fertilizer in Indiana – research update. Purdue University Extension Entomology Pest & Crop Newsletter 2023.1. https://extension.entm.purdue.edu/newsletters/pestandcrop/article/corn-response-to-sulfur-fertilizer-in-indiana-research-update-2/
ECCC. (2024). Air quality agreement − progress report 2020-2022. Environment and Climate Change Canada. https://www.canada.ca/en/environment-climate-change/services/air-pollution/issues/transboundary/canada-united-states-air-quality-agreement-overview/report-2020-2022.html
Fleuridor, L., Fulford, A., Lindsey, L. E., Lentz, E., Watters, H., Dorrance, A., Minyo, R., Richer, E., Chaganti, V., Kumaran, S., & Culman, S. W. (2023). Ohio grain crop response to sulfur fertilization. Agronomy Journal, 115, 2007–2016. https://doi.org/10.1002/agj2.21328
GFO. (2024). Response of corn, soybean and cereals to sulphur application. Grain Farmers of Ontario. https://gfo.ca/research-projects/c2018ago6/
Heckman, J.R., Sims, J.T., Beegle, D.B., Coale, F.J., Herbert, S.J., Bruulsema, T.W. & Bamka, W.J. (2003), Nutrient Removal by Corn Grain Harvest. Agron. J., 95, 587-591. https://doi.org/10.2134/agronj2003.5870
Hinckley, EL. S., Crawford, J.T., Fakhraei, H., & Driscoll, C.T. (2020). A shift in sulfur-cycle manipulation from atmospheric emissions to agricultural additions. Nature Geoscience, 13(9), 597–604. https://doi.org/10.1038/s41561-020-0620-3
Hinckley, EL.S., & Driscoll, C.T. (2022). Sulfur fertiliser use in the Midwestern US increases as atmospheric sulfur deposition declines with improved air quality. Communications Earth & Environment, 3(1), 1–5. https://doi.org/10.1038/s43247-022-00662-9
Kaiser, D.E., Sutradhar, A.K. & Wiersma, J.J. (2019). Do hard red spring wheat varieties vary in their response to sulfur? Agronomy Journal, 111, 2422-2434. https://doi.org/10.2134/agronj2018.12.0798
Ma, B.-L., Biswas, D.K., Herath, A.W., Whalen, J.K., Ruan, S.Q., Caldwell, C., Earl, H., Vanasse, A., Scott, P. & Smith, D.L. (2015), Growth, yield, and yield components of canola as affected by nitrogen, sulfur, and boron application. Journal of Plant Nutrition and Soil Science, 178, 658-670. https://doi.org/10.1002/jpln.201400280
NADP. (2024). Total Deposition Science Committee, Total Deposition Maps. National Atmospheric Deposition Program. https://nadp.slh.wisc.edu/committees/tdep/#tdep-maps
Putman, J. L., Ketterings, Q., Cherney, J. H., & Overton, T. R. (2021). Impact of sulfur application on soybean yield and quality in New York. Agronomy Journal, 113, 2858–2871. https://doi.org/10.1002/agj2.20690
The Fertilizer Institute. (2020). Soil fertility manual. The Fertilizer Institute.
The Fertilizer Institute. (2021). Soil test levels in North America, 2020 summary update. The Fertilizer Institute. https://soiltest.tfi.org/
Self-Study CEU Quiz
Earn 1 CEU in Nutrient Management by taking the quiz for the article at https://web.sciencesocieties.org/Learning-Center/Courses. For your convenience, the quiz is printed below. The CEU can be purchased individually, or you can access as part of your Online Classroom Subscription.
- Sulfur forms part of the atomic structure of the amino acid
- glutamine.
- lysine.
- methionine.
- tyrosine.
- Except that they start on younger leaves first, symptoms of sulfur deficiency are most like those of
- boron.
- calcium.
- nitrogen.
- potassium.
- Sulfur deposition to cropland from the atmosphere decreased dramatically from the years 1990-2000 to the years 1999-2000. The primary reason was
- climate change.
- COVID-19 pandemic.
- emission controls on fuels.
- increased crop removal of sulfur.
- One jurisdiction that showed less frequent response to sulfur than was expected owing to reduced deposition from the atmosphere in recent years was
- Indiana.
- Ohio.
- Ontario.
- Pennsylvania.
- One advantage to using a thiosulfate form of sulfur fertilizer, as compared to ammonium sulfate, is that it
- can inhibit nitrous oxide emission.
- facilitates more rapid nitrification.
- is more immediately available to the plant.
- is safer to place with the seed.
- Agronomic interpretations of soil tests for sulfur are not very clear because
- not enough samples are taken.
- more soil samples are testing low.
- sandy soils show low response to sulfur.
- soil tests poorly predict crop response.
- The fertilizer source that contains the highest percentage of sulfur is
- ammonium sulfate.
- ammonium thiosulfate.
- ammonium polysulfide.
- elemental sulfur.
- A sulfur fertilizer source that generates acidity and may lower soil pH is
- ammonium sulfate.
- gypsum.
- magnesium sulfate.
- potassium sulfate.
- Soil degradation arising from the acidity generated by nitrogen and sulfur fertilizers may be mitigated by applying
- acid rain.
- elemental sulfur.
- gypsum.
- lime.
- By reducing nitrification, thiosulfates may not only reduce emissions of nitrous oxide, but also
- reduce nitrate leaching.
- increase soil pH.
- reduce soil carbon.
- reduce crop yield.
Text © . The authors. CC BY-NC-ND 4.0. Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.










